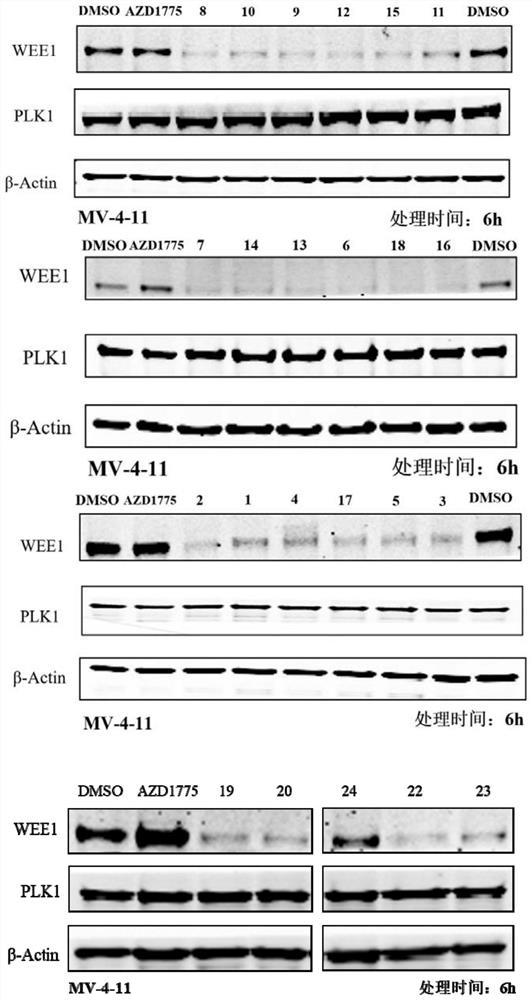
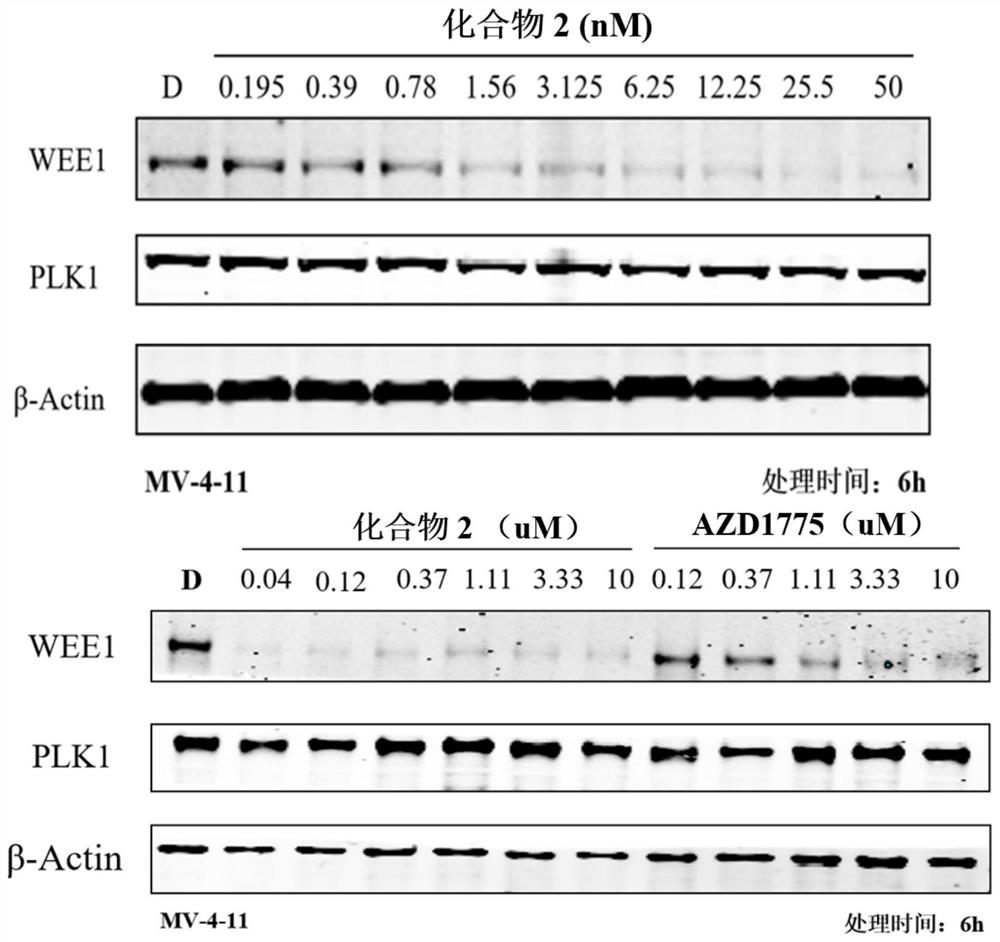
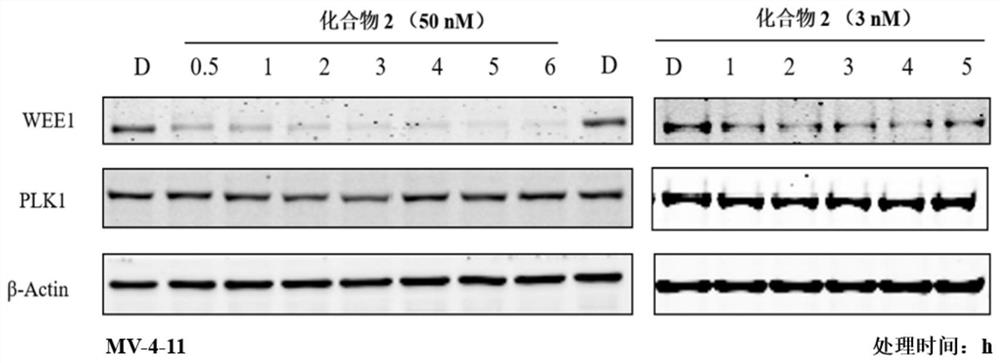
WEE1 protein degradation agent
A protein kinase, C1-C6 technology, applied in the field of chemistry, can solve the problems of genome instability chromosome, mitotic catastrophe, clinical toxicity and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0301] Embodiment 1: the synthetic route of intermediate S15
[0302] Step 1: Synthesis of Compound S3
[0303]
[0304] Compound S1 (10.0 g, 67.5 mmol) and compound S2 (9.40 g, 70.9 mmol) were added to 110 mL of toluene solution, the reaction system was heated to reflux, and reacted for 18 hours. After the detection reaction was completed, it was cooled to room temperature, filtered, washed with n-hexane, and the white solid product was collected and spin-dried to obtain compound S3 (16.1 g, 91% yield). 1 H NMR (400MHz, DMSO) δ 9.87 (s, 1H), 7.95 (d, J=3.2Hz, 4H), 1.45 (s, 9H).
[0305] Step 2: Synthesis of Compound S5
[0306]
[0307] Compound S3 (16.1g, 61.2mmol), potassium carbonate (16.1g, 116mmol) and benzyltriethylammonium chloride (1.39g, 6.12mmol) were added to 110mL of acetonitrile solvent and stirred for 5 minutes, then compound S4 (8mL ,91.8mmol) was added into the reaction system and reacted at room temperature for 18 hours. After the detection reaction...
Embodiment 2
[0330] Embodiment 2: the general synthetic route of compound 1-3
[0331]
[0332] The synthetic route of compound 1:
[0333] Step 1: Synthesis of Compound S18
[0334]
[0335] Compounds S13 (250 mg, 0.51 mmol) and S17 (148 mg, 0.62 mmol) were added to 10 mL of DMF solution. After the system was completely dissolved, potassium carbonate (141 mg, 1.02 mmol) was added, and the temperature was raised to 60°C for 6 hours. After the reaction was completed, 100 mL of water was added, extracted with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was collected and concentrated to obtain a crude product. The crude product was separated and purified by silica gel column chromatography to obtain yellow solid compound S18 (220 mg, 67% yield). 1 H NMR(400MHz,DMSO)δ10.17(s,1H),8.83(d,J=1.0Hz,1H),8.05(s,1H),7.75(d,J=7.3Hz,1H),7.65-7.46 (m,3H),6.92(d,J=7.6Hz,2H),6.83(s,1H),5.66(dd,J=14.5,8.9Hz,1H),5.33(s,1H),4.99(d, J=10...
Embodiment 3
[0344] Embodiment 3: the general synthetic route of compound 4-7
[0345]
[0346] The synthetic route of compound 4:
[0347] Step 1: Synthesis of compound S21
[0348]
[0349] Compounds S16 (1 g, 3.62 mmol) and S20 (630 mg, 3.62 mmol) were added to 10 mL of DMF solution, DIPEA (1.5 mL) was added under nitrogen protection, and the reaction system was heated to 90°C for 12 hours. After the reaction was completed, 100 mL of water was added to the system, extracted with ethyl acetate, the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was collected and concentrated to obtain a crude product. The crude product was separated and purified by silica gel column chromatography to obtain yellow solid compound S21 (1.17 g, 75% yield). 1 H NMR (400MHz, DMSO) δ11.09(s, 1H), 7.57(t, J=7.3Hz, 1H), 7.08(d, J=8.4Hz, 1H), 7.02(d, J=6.6Hz, 1H ),6.91(s,1H),6.66(s,1H),5.05(d,J=9.1Hz,1H),3.29(t,J=12.3Hz,2H),3.00(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com