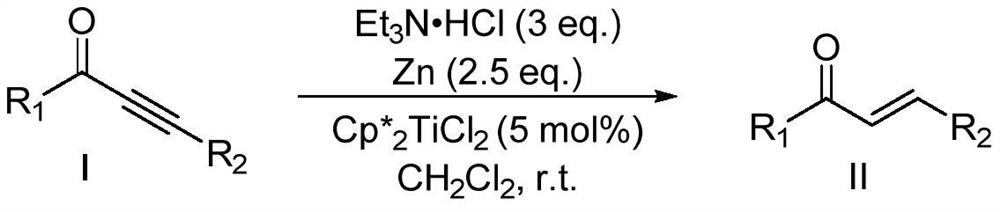Method for preparing chalcone compound by catalyzing selective hydrogenation reduction of acetylenic ketone with Ti (III) complex
A technology for chalcones and catalyzing alkynones, which is used in organic compound/hydride/coordination complex catalysts, preparation of organic compounds, preparation of carbon-based compounds, etc., to achieve a wide range of biological activity and medicinal value, reaction products Single, mild reaction conditions for effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
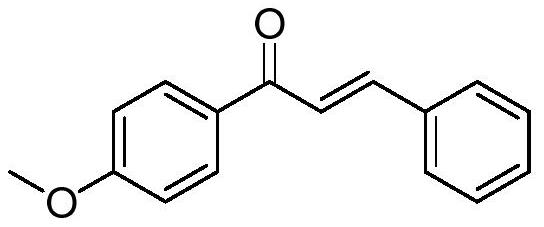
[0015] Preparation of (E)-1-(4-methoxyphenyl)-3-phenylprop-2-en-1-one of the following structural formula:
[0016]
[0017] Add 0.103g (0.75mmol) Et to 20mL Shrek in the glove box 3 NHCl, 40mg (2.5eq) zinc powder, 0.0045g (5%mol) Cp * 2 TiCl 2 , 3mL of dichloromethane, stirred and reacted at room temperature for 10 minutes to prepare a trivalent titanium system, and dissolved 1-(4-methoxyphenyl)-3-phenylprop-2-yn-1-one in 2mL Dichloromethane was then added to the trivalent titanium system, reacted at room temperature for 6h, and the dichloromethane was removed by rotary evaporation, separated with a silica gel column (the eluent was a mixture of ethyl acetate and petroleum ether with a volume ratio of 1:400), (E)-1-(4-Methoxyphenyl)-3-phenylprop-2-en-1-one was obtained in 86% yield.
[0018] The resulting product is characterized by a 400MHz nuclear magnetic resonance spectrometer (JEOL), and the characterization data are: 1 H NMR (400MHz, CDCl 3 )δ8.20(d, J=8.8Hz, 2...
Embodiment 2
[0020] Preparation of (E)-1-(4-methoxyphenyl)-3-(p-tolyl)propyl-2-en-1-one of the following structural formula:
[0021]
[0022] In Example 1, the 1-(4-methoxyphenyl)-3-phenylprop-2-yn-1-one used was used with equimolar 1-(4-methoxyphenyl)-3- (P-tolyl) prop-2-yne-1-ketone replacement, other steps are the same as in Example 1, to obtain (E)-1-(4-methoxyphenyl)-3-(p-tolyl)propyl- 2-en-1-one in 80% yield.
[0023] The resulting product is characterized by a 400MHz nuclear magnetic resonance spectrometer (JEOL), and the characterization data are: 1 H NMRδ8.04(d, J=8.9Hz, 2H), 7.79(d, J=15.6Hz, 1H), 7.59-7.47(m, 3H), 7.22(d, J=8.0Hz, 2H), 6.98( d,J=8.9Hz,2H),3.89(s,3H),2.39(s,3H); 13 C NMR (101MHz, Chloroform-d) δ187.8, 162.3, 143.0, 139.8, 131.3, 130.2, 129.7, 128.6, 127.4, 119.8, 112.8, 54.5, 20.5.
Embodiment 3
[0025] Preparation of (E)-3-(4-bromophenyl)-1-(4-methoxyphenyl)propyl-2-en-1-one of the following structure:
[0026]
[0027] In Example 1, the 1-(4-methoxyphenyl)-3-phenylprop-2-yn-1-one used was mixed with equimolar 3-(4-bromophenyl)-1-(4 -Methoxyphenyl propane)-2-yn-1-ketone replacement, other steps are the same as in Example 1, to obtain (E)-3-(4-bromophenyl)-1-(4-methoxybenzene base) propyl-2-en-1-one in 88% yield.
[0028] The resulting product is characterized by a 400MHz nuclear magnetic resonance spectrometer (JEOL), and the characterization data are: 1 H NMR (400MHz, Chloroform-d) δ8.08-7.97 (m, 2H), 7.72 (d, J = 15.7Hz, 1H), 7.57-7.45 (m, 5H), 7.02-6.93 (m, 2H), 3.88(s,3H); 13 C NMR (101MHz, Chloroform-d) δ187.3, 162.5, 141.5, 133.0, 131.1, 129.8 (d, J=4.5Hz), 128.7, 123.5, 121.3, 112.9, 54.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com