Method for preparing hindered amine nitroxide free radical compound by alkaline heterogeneous catalysis system
A hindered amine compound, heterogeneous catalysis technology, applied in the direction of organic chemistry and the like, can solve the problems that the preparation method cannot be simple, the purity requirement is high, and the catalyst cost is high, and achieves easy post-processing, high yield, and simple preparation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
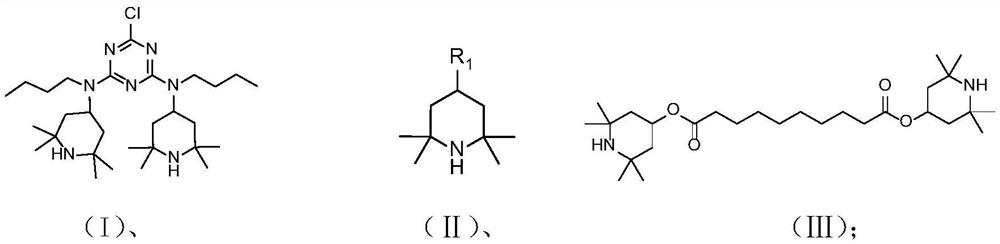
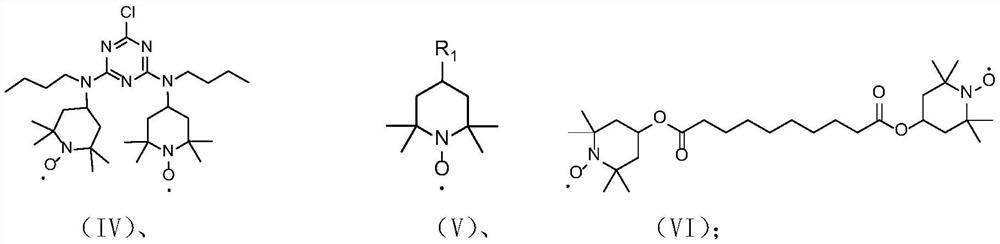
[0039] Alkaline non-uniform catalytic system preparation preparation of gamlamine nitrogen oxygen free radical compound (2-chloro-4,6-double (2,2,6,6-tetramethylpioadhoxy radical-4-butyl) Method of triazine)
[0040] Take 2 g (4 mmol) 2-chloro-4,6-bis (2,2,6,6-tetramethylpiperidine-4-butyl amino) triazine I added to 50 ml of two round bottom flasks, assembly thermometer The sleeve is added 10 ml of toluene to the reaction container to dissolve the compound of formula I, and maintain the reaction system at 30 ° C, while continuously stirring, the reaction system is adjusted by 0.2 g / mL of sodium carbonate solution to 10.3, slow drop 0.8 mL 50% (mass concentration) hydrogen peroxide (20 mmol), reaction, TLC indicates the end point (about 20h), stopped the reaction, phase phase, and dry the organic phase after drying with anhydrous sodium sulfate, concentrated organic phase to obtain a goal The compound of the product formula IV 2.24 g red solid, yield is 99%, purity 99%.
[0041] ...
Embodiment 2
[0043] Method for preparing an alkaline non-uniform catalytic system (2,2,6,6-tetramethylpiperidine-4-alcohol nitrogen oxyfitride)
[0044] 2 g (12 mmol) 2,2,6,6-tetramethylpiperidine-4-alcohol II-1 was added to 50 ml of two round bottom flasks, assembled the thermometer sleeve, and 10 ml of ethyl acetate was added to the reaction vessel. The reaction solvent dissolves the compound of the II-1, and the reaction system is maintained at 30 ° C, and the reaction system is continuously stirred, and the reaction system pH is 9.0, and 2.4 ml of 30% (mass concentration) is adjusted slowly. The hydrogen peroxide (24 mmol) was carried out, the TLC indicates the end point (about 6 h), stopped the reaction, phase-phase, after collecting the organic phase, dried over anhydrous sodium sulfate, concentrated organic phase to obtain a target product V-1 2.04 g of red solid, yield is 99%, purity 98%.
[0045]
Embodiment 3
[0047] Method for preparing an alkaline non-apex catalytic system (2,2,6,6-tetramethylpiperidine-4-ketozo-free radical)
[0048] 2 g (12 mmol) 2,2,6,6-tetramethylpiperidine-4-ketone (II-2) was added to 50 ml of two round bottom flasks, fitting a thermometer sleeve, and 10 ml of reaction solvent was added to the reaction vessel. (5 ml of xylene, 5 ml of ethyl acetate) dissolves the compound of formula II-2, and maintains the reaction system at 40 ° C, while continuously stirring, using 0.01 g / ml of potassium hydrogencarbonate aqueous solution, regulating the reaction system pH 7.5, The reaction was slowly added dropwise to the hydrogen peroxide (36 mmol) of hydrogen peroxide (36 mmol), TLC indicates the end point (about 6h), stopped the reaction, phase-phase, and dry the organic phase after drying, concentrated organic phase The compound 1.99 g of the compound of the Target product V-2 was obtained, and the yield was 97%, purity 99%.
[0049]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com