Application of acetal phosphatidyl ethanolamine in preparation of product for reducing trimethylamine oxide in vivo
A technology of plasmalogen phosphatidylethanolamine and trimethylamine oxide, applied in medical preparations containing active ingredients, food science, organic active ingredients, etc., can solve the problems of no research attention, achieve broad market prospects, and reduce the level of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
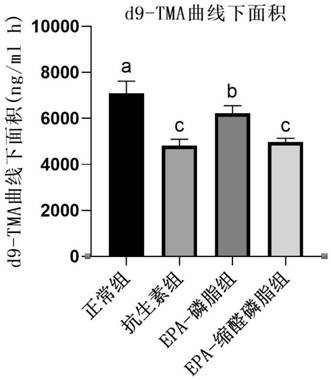
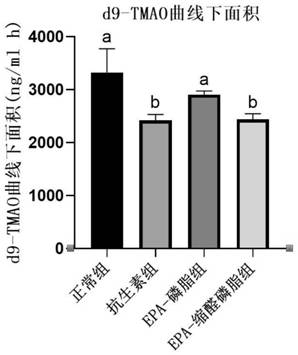
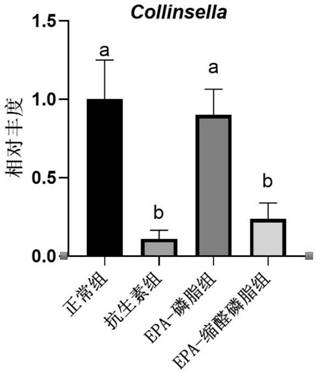
[0026] Embodiment 1: Effect of plasmalogen phosphatidylethanolamine on content of trimethylamine oxide in mice
[0027] 1.1 Experimental method
[0028] 1.1.1 Experimental design
[0029] ICR mice were divided into 5 groups: normal group, antibiotic group, EPA-phospholipid group, EPA-plasma phosphatidylethanolamine group. The normal control group was given drinking water and common rod-shaped feed; the antibiotic group was given mixed antibiotic solution (1 mg / ml ampicillin and 0.5 mg / ml neomycin sulfate) and common rod-shaped feed, and the antibiotic water was changed every two days; the EPA-phospholipid group Drinking water and rod-shaped feed containing 1% EPA-phosphatidylethanolamine; EPA-plasma-phosphatidylethanolamine group were given drinking water and rod-shaped feed containing 1% EPA-plasma-phosphatidylethanolamine. After 20 days of intervention with the test substance, 45 μM d9-choline chloride was administered to the stomach, blood was collected from the tail vein...
Embodiment 2
[0045] Embodiment 2: Effect of plasmalogen phosphatidylethanolamine on content of trimethylamine oxide in mice
[0046] 2.1 Experimental method
[0047] 2.1.1 Experimental design
[0048] ICR mice were divided into three groups: normal group, sphingomyelin group, EPA-plasma phosphatidylethanolamine group. The normal group received drinking water and common rod-shaped feed; the sphingomyelin group received drinking water and rod-shaped feed containing 1% sphingomyelin; feed. After 30 days of intervention with the test substance, 45 μM d9-choline chloride was administered into the stomach, blood was collected from the tail vein, and the concentrations of d9-TMA and TMAO in serum at different time points were detected by LC-MS.
[0049] 2.1.2 Sample pretreatment
[0050] Take 20uL of serum, add 4 times the volume of cold methanol, vortex and shake for 30s, and sonicate in an ice-water bath for 5min. After ultrasonication, centrifuge at 12000rpm for 15min, take 50uL of the su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com