Fluorescent probe based on benzothiazole fluorophore and application of fluorescent probe in hypochlorous acid detection
A fluorescent probe, benzothiazole technology, applied in the field of fluorescent probe based on benzothiazole fluorophore and in the detection of hypochlorous acid, achieving obvious results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Fluorescent probe preparation:
[0039] Add 3mmol of salicylaldehyde and 3.2mmol of 2-aminomercaptophenol into a round bottom flask filled with 6mL of ethanol solution, and connect a glass three-way valve with a balloon attached to the flask. Vacuumize the flask and pass nitrogen through a glass three-way valve with a balloon, and react at room temperature for 30 minutes. After reacting for 30 min, quickly add 0.34 mL of H 2 o 2 (30%, mass) and 0.17 mL of HCl (37%, mass). Continue to react under nitrogen atmosphere for 12h.
[0040] After the reaction was over, the flask was kept under ice-water conditions with 0.05 mL of HCl (37%), then the solution was transferred to a 100 mL beaker, and 30 mL of saturated saline was added. Extracted 3 times with 50 mL of ethyl acetate, and evaporated the sample to dryness by a rotary evaporator to obtain a preliminary purified sample.
[0041] The preliminarily purified sample was dissolved in ethyl acetate and an appropriate am...
Embodiment 2
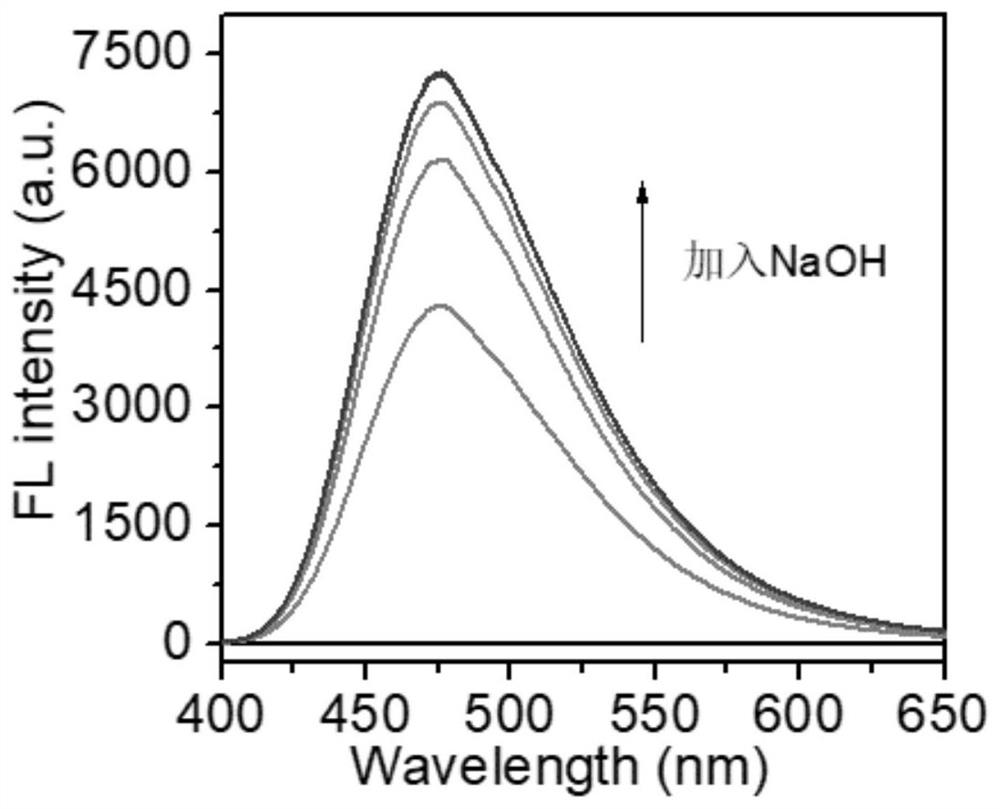
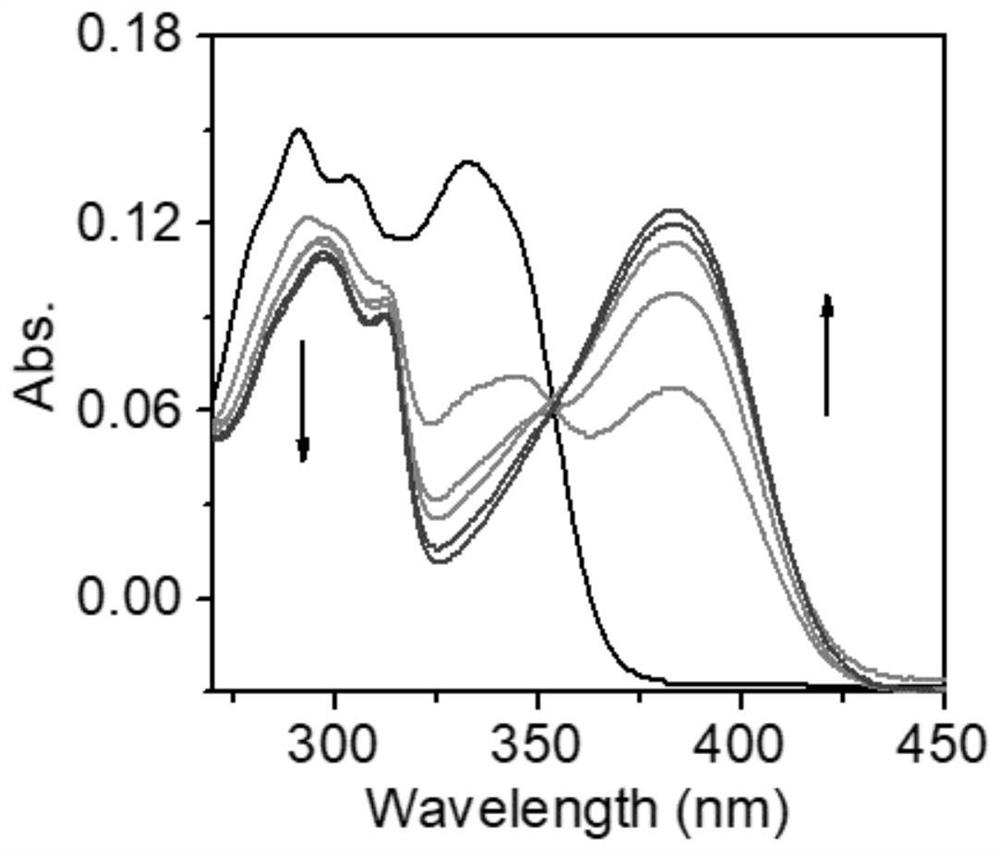
[0043] 0.01 mmol of the probe was dissolved in 10 mL of methanol solution to obtain a 1 mM probe stock solution. Take 20 μL of the probe stock solution and add it to the cuvette of methanol and aqueous solution, then gradually add 5, 10, 15, 20 and 25 μL of 0.04M sodium hydroxide solution. When it was added to 20 μL, the fluorescence intensity basically stopped increasing. Therefore, 20 μL of NaOH solution with a concentration of 0.04 M was added under the same conditions for subsequent solvent environments. And at the same time, the corresponding ultraviolet absorption spectrum is measured and added. Such as figure 1 , figure 2 shown.
Embodiment 3
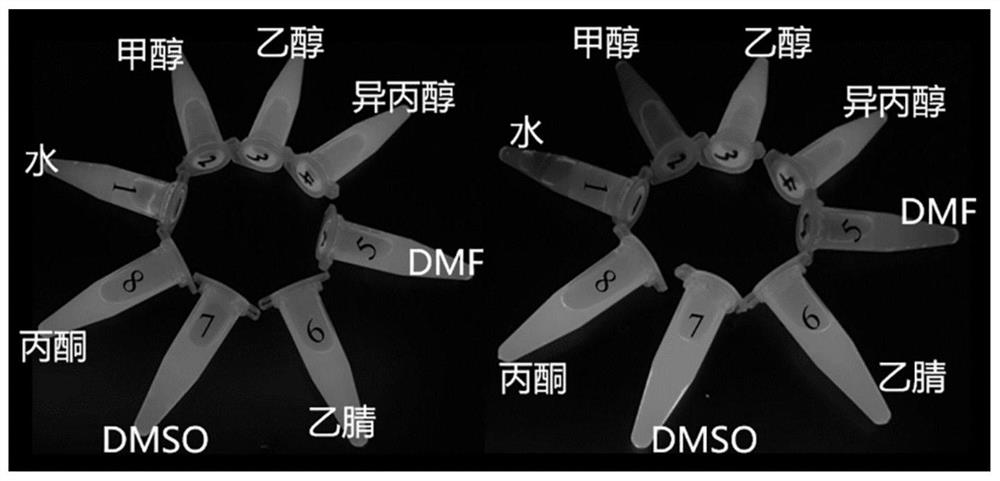
[0045] Take 20 μL of the probe stock solution described in Example 2 and add it to the centrifuge tubes of water, methanol, ethanol, isopropanol, N,N-dimethylformamide, acetonitrile, dimethyl sulfoxide and acetone solvents respectively , and then add 20 μL of NaOH solution with a concentration of 0.04M, and then add the same and excess sodium hypochlorite solution, and take fluorescence pictures under a UV lamp with a wavelength of 365 nm. It was found that in methanol solution, the quenching effect of the probe was the best (obvious fluorescence quenching). Such as image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com