Docetaxel micelle nano-drug as well as preparation method and application thereof
A docetaxel and micellar nanotechnology, which is applied in drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problem that the prolongation of patient survival does not meet expectations, and achieve the reduction of enrichment, increase of solubility, and increase of cycle time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
[0031] Synthesis Example Synthesis of polymers is a conventional method
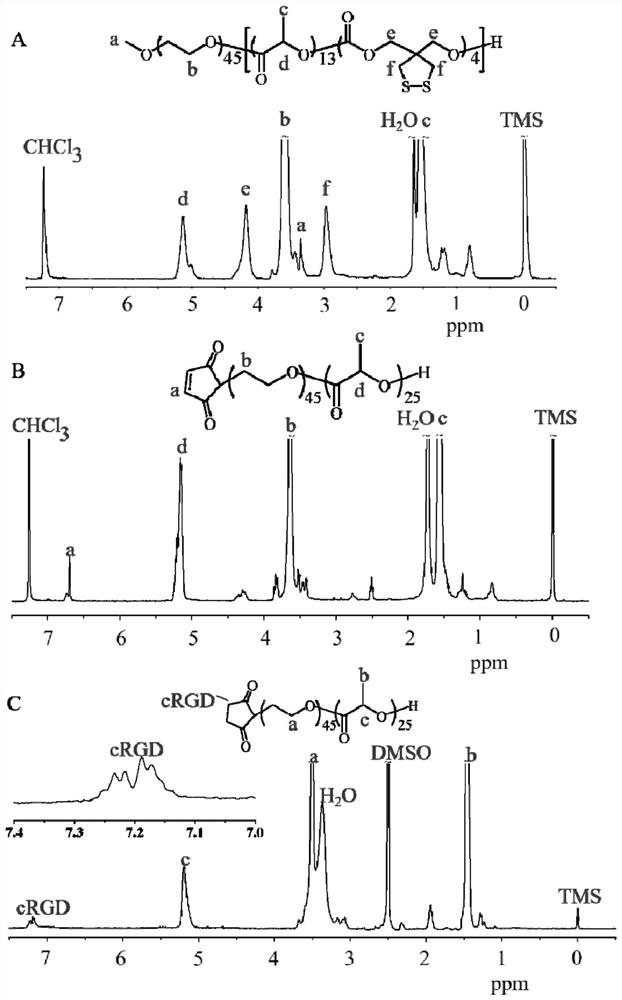
[0032] PEG-P(LA-DTC) was synthesized by ring-opening polymerization of DTC and LA in anhydrous DCM with PEG as macroinitiator and DBU as catalyst. For example, stir MeO-PEG-OH (0.4 g, 200 μmol), DTC (0.14 g, 7.1 mmol), and LA (0.2 g, 13.9 mmol) in DCM (10 mL) in a glove box under nitrogen atmosphere until completely dissolved. DBU (30 mg, 2 mmol) was added under stirring, then the reactor was sealed and transferred out of the glove box, and placed in an oil bath at 37°C for 3 hours to react. Then the reaction solution was added dropwise to a 20-fold excess of cold anhydrous ether to precipitate, after centrifugation, it was dissolved with acetonitrile, and the precipitation was repeated twice. The obtained PEG-P (LA-DTC) was vacuum-dried for 24 hours to obtain a light yellow Lumpy solid. Yield: 76%. polymer through 1 H NMR and GPC measure relative molecular weight and molecular weight distribution. ...
Embodiment 1
[0041] Example 1 Preparation and Characterization of DTX Micellar Nanomedicine
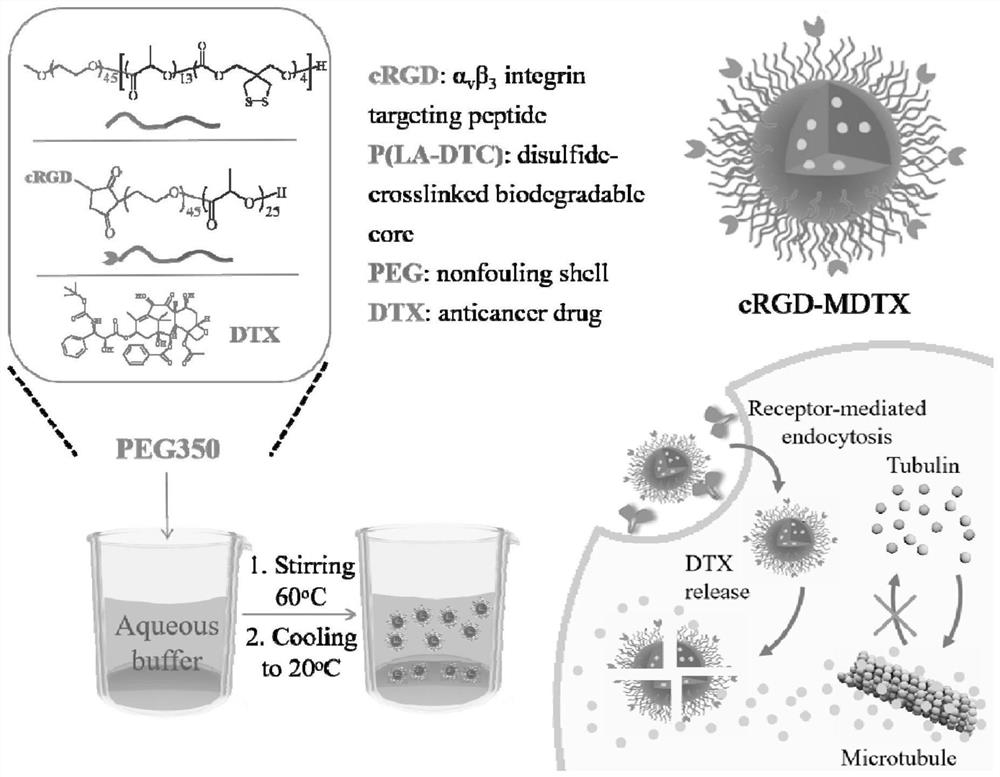
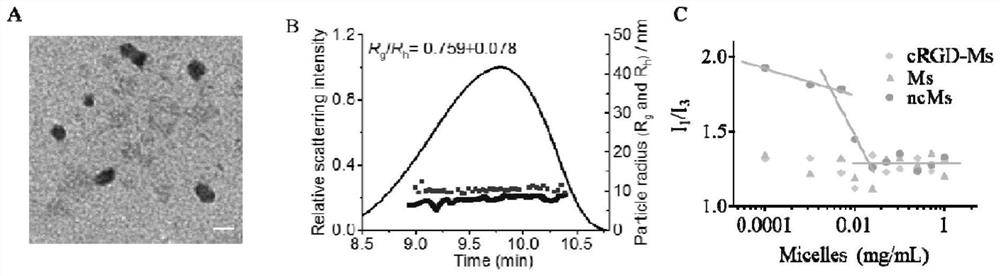
[0042] PEG 2k -P(LA 1k -DTC 0.7k ), cRGD-PEG 2k -PLA 1.7k and DTX were dissolved in PEG350 at concentrations of 200 mg / mL, 50 mg / mL and 100 mg / mL to configure three mother solutions. After mixing evenly according to the designed ratio, take 0.05mL of the mixed solution and heat it to 60°C and inject it into 0.95mL phosphate buffer (PB, 10 mM, pH 7.4) preheated to 60°C to obtain a uniform and clear gel. The beam solution cRGD-MDTX is a surface-coupled cRGD, PEG-P (LA-DTC)-based disulfide cross-linked, small-sized DTX micellar nanomedicine.
[0043] By changing the ratio of the three mother solutions, a series of micelles with different cRGD density and DTX drug loading can be obtained. Among them, cRGD-PEG-PLA and PEG-P (LA-DTC) are mixed according to the molar ratio of 0 / 100, 2.5 / 97.5, 5 / 95, and 10 / 90, and the cRGD surface density can be obtained as 0, 2.5%, 5%, respectively. % and 10% mice...
Embodiment 2
[0056] Example 2 Cellular uptake experiment of DTX micellar nanomedicine
[0057] PC3 cells were used. In the flow cytometry (FACS) test, first spread 2 mL of PC3 cells in a 6-well plate (3×10 5 cells / well) for 24 hours. After adding 200 µL of Cy5-MDTX, 2.5% cRGD / Cy5-MDTX, 5% cRGD / Cy5-MDTX or 10% cRGD / Cy5-MDTX and incubating for 4 hours, the medium and micelles were removed. After washing with PBS, add 0.25% (w / v) trypsin and 0.03% (w / v) EDTA to digest to a single cell suspension, centrifuge the cell suspension at 1000 rpm for 3 minutes, wash with PBS twice, and finally suspend the cells In 500 µL PBS, the fluorescence intensity of cell-associated Cy5 was detected by FACS. MCF-7 cells were used as negative control. In confocal laser microscopy (CLSM) testing, PC3 cells were plated in 24-well plates (3.0 × 10 5 cells / well) for 24 hours. Then add 200 µL of 5% cRGD / Cy5-MDTX or Cy5-MDTX and continue to culture for 4 hours. After removing the culture medium, fix with 4% form...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com