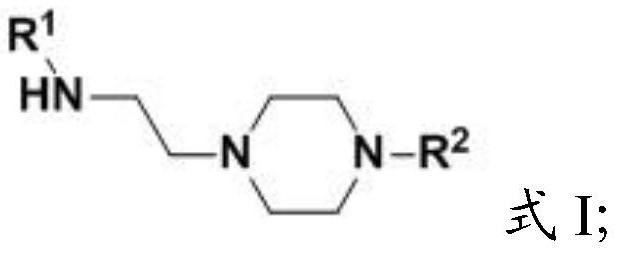
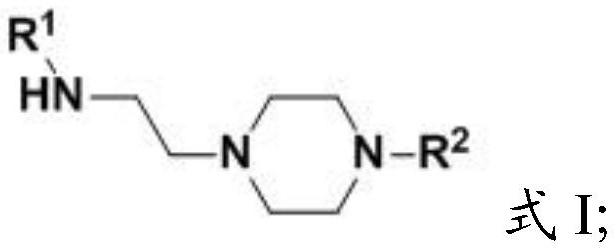
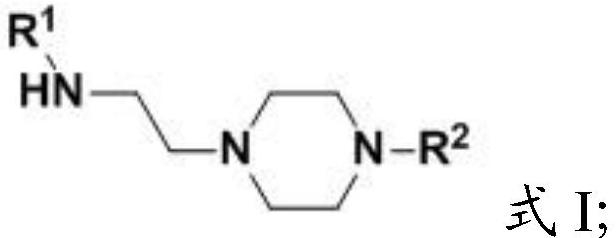
Aminoethylated piperazine and preparation method thereof, carbon dioxide absorbent and application of carbon dioxide absorbent
A technology of carbon dioxide and amine ethyl, which is applied in chemical instruments and methods, separation methods, gas treatment, etc., can solve the problems of poor thermal stability, unsatisfactory recycling performance, degradation and inactivation of alcohol amine absorbents, and achieve improved Recycling efficiency, enhanced stability, and low energy consumption for desorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The present invention provides the preparation method of the amine ethylated piperazine described in the above technical scheme, (i) when the R 2 When it is a C1-C4 alkyl group, the preparation method includes the following steps:
[0046] Mixing compound 1, compound 2 and water for substitution reaction to obtain aminoethylated piperazine;
[0047] Among them, R 1 It is C1~C4 alkyl;
[0048] (ii) when the R 2 for When, the preparation method comprises the following steps:
[0049] Mixing compound 3, compound 4 and water for substitution reaction to obtain aminoethylated piperazine;
[0050] Among them, R 1 , R 3 and R 4 are independently C1-C4 alkyl groups.
[0051] In the present invention, unless otherwise specified, all raw material components are commercially available products well known to those skilled in the art.
[0052] When the R 2 When it is a C1-C4 alkyl group, the preparation method includes the following steps: mixing compound 1, compound...
Embodiment 1
[0088] Synthesis of N-(2-methylaminoethyl)-N'-methyl-piperazine
[0089]
[0090] Step 1: In a 500mL single-necked bottle, dissolve 240mmol of triethanolamine in 350mL of dichloromethane, and stir at room temperature and 600r / min for 10min. Under cooling in an ice-water bath, 1200 mmol thionyl chloride was added dropwise to the solution at a constant speed using a constant-pressure dropping funnel, and the dropwise addition was completed within 30 minutes. Immediately, the one-necked bottle was transferred to an oil bath at 40° C., and refluxed for 12 hours. Then the solvent dichloromethane and excess thionyl chloride were removed by a rotary evaporator to obtain the intermediate tris(2-chloroethyl)amine hydrochloride.
[0091] Step 2: Transfer all the tris(2-chloroethyl)amine hydrochloride obtained in step 1 to a 500mL autoclave, add 260mL of a 40wt% methylamine aqueous solution, seal it and place it in a 60°C oil bath, 800r / h React for 12 hours under the condition of st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com