Method for preparing, separating and purifying biphenyl derivative through catalytic coupling
A technology of derivatives and biphenyl, which is applied in the field of synthetic chemistry, catalytic coupling preparation and separation and purification of biphenyl derivatives, can solve the problems affecting the reaction process and difficult to reduce, and achieve the effect of simple method of purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
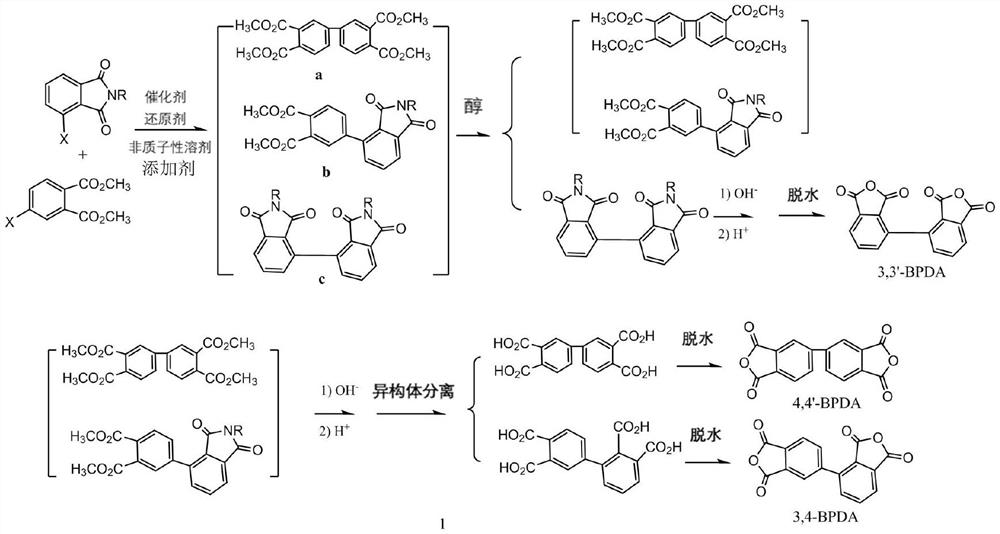
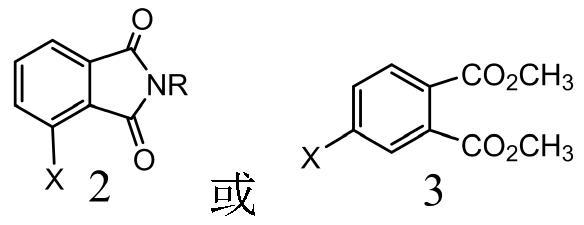
[0028] Take a 500mL three-neck flask, put dimethyl 4-chlorophthalate (22.8g, 0.1moL), N-phenyl-3-chlorophthalimide (25.7g, 0.1moL), zinc powder (6.5g, 0.1moL), anhydrous NiCl 2 (255mg, 2mmoL) and triphenylphosphine (0.5g, 2mmoL), mixed in a nitrogen atmosphere, added 145mL of anhydrous DMAc, 50 ℃ ultrasonically assisted stirring reaction for 4 hours, filtered the insoluble matter, and the obtained filtrate was decompressed in a round bottom flask 125 mL of the solvent DMAc was recovered to obtain a viscous solid.
[0029] 1) Add 150g of ethanol to the system and reflux for 30 minutes, filter out the insoluble matter N-phenyl-N'-phenyl-2,2',3,3'-biphenylbisimine (collectively: 3,3 '-biphenylbisimine), dried at 100°C for 10 hours to obtain 10 g of 3,3'-biphenylbisimine, added 24 g of 20% aqueous sodium hydroxide solution to a 200 mL reaction flask, heated to reflux for 24 hours, filtered off Insoluble matter, cooled, adjusted pH=1 with concentrated hydrochloric acid, cooled to...
Embodiment 2
[0033]Take a 500mL three-neck flask, put dimethyl 4-chlorophthalate (22.8g, 0.1moL), N-methyl-3-chlorophthalimide (9.8g, 0.05moL), zinc powder (5.2g, 0.08moL), anhydrous NiCl 2 (255mg, 2mmoL) and triphenylphosphine (3.5g, 14mmoL), mixed in a nitrogen atmosphere, added 160mL of anhydrous DMAc, 500w ultrasonic assisted 100 ℃ stirring reaction for 1 hour, filtered the insoluble matter, and the obtained filtrate was reduced in a round bottom flask 140 mL of solvent DMAc was recovered under pressure.
[0034] 1) Add 100g of ethanol to the system and reflux for 30 minutes, all of which are dissolved, and no 2,2',3,3'-biphenylbisimine is formed. All the mixture in the system is a mixture of 4,4'-biphenyl tetramethyl ester and N-methyl-34'-biphenylimine dimethyl ester, distilled off to recover ethanol, and add 20% hydroxide to the phase system at the same time Sodium aqueous solution 64g, heated to reflux for 12 hours, filtered off insoluble matter, cooled, and adjusted to pH=1 with...
Embodiment 3
[0036] Take a 500mL three-neck flask, put dimethyl 4-chlorophthalate (22.8g, 0.1moL), N-methyl-3-chlorophthalimide (19.5g, 0.1moL), zinc powder (13g, 0.2moL), anhydrous NiCl 2 (510mg, 4mmoL) and 2,2-bipyridine (2.18g, 14mmoL), mixed in a nitrogen atmosphere, then added 140mL of anhydrous NMP, 400w ultrasonic assisted stirring reaction at 90 ° C for 2 hours, filtered the insoluble matter, and the obtained filtrate was in the round bottom The solvent NMP 120mL was recovered under reduced pressure in the flask to obtain a viscous solid.
[0037] 1) Add 120 g of ethanol to the obtained viscous solid, reflux for 30 minutes, filter out the insoluble matter while hot to obtain N-methyl-N'-methyl-2,2',3,3'-biphenylbisimine ( Collectively: 3,3'-biphenylbisimine) 7.5g, alcohol mother liquor for later use. Add 20g of 20% sodium hydroxide aqueous solution to 3,3'-biphenylbisimine, heat and reflux for 24 hours, filter off insoluble matter, cool, adjust pH=1 with concentrated hydrochloric...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com