Application of bacteroides cellulosilyticus in prevention and/or treatment of inflammatory bowel diseases
A technology of inflammatory bowel disease and cellulose, applied in the field of microorganisms, can solve the problems that the application has not been disclosed, and achieve the effect of great application value, no toxic side effects, and excellent resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 The cultivation of Bacteroides cellulolyticum
[0055] Streak activated strain: DSM 14838T strain of Bacteroides cellulolyticum, used BHA (brain heart infusion agar medium) plate for streak activation. Anaerobic culture at 37°C for 2-7 days to obtain a single colony.
[0056] Colony characteristics: After Bacteroides cellulolyticum was cultured on the BHA plate for 48 hours, the colony characteristics were round, slightly convex, translucent, white, and smooth.
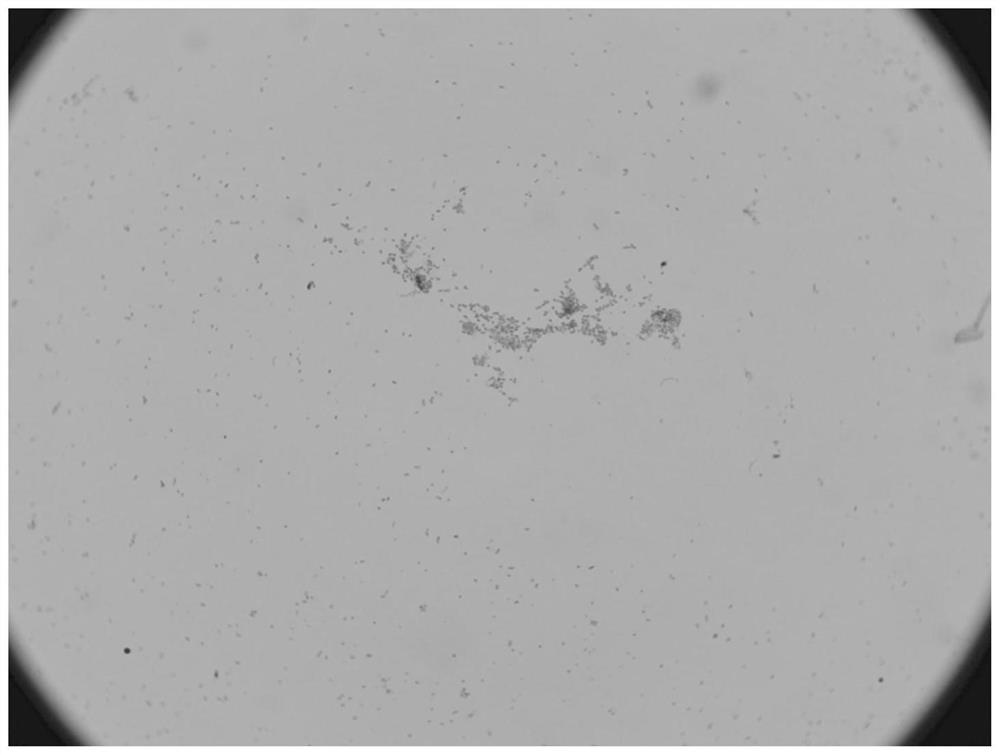
[0057] Morphology under the microscope: Bacteroides cellulolyticum was subjected to Gram-staining microscopic examination, and it was a Gram-negative bacterium with a typical rod shape, blunt rounded ends and dense staining, and the uncolored part in the middle of the bacteria was like a vacuole. figure 1 .
[0058] Sludge preparation: select a single colony and inoculate it in tryptone broth for 8 hours of fermentation (at a temperature of 37°C), centrifuge the obtained bacterial solution at a spee...
Embodiment 2
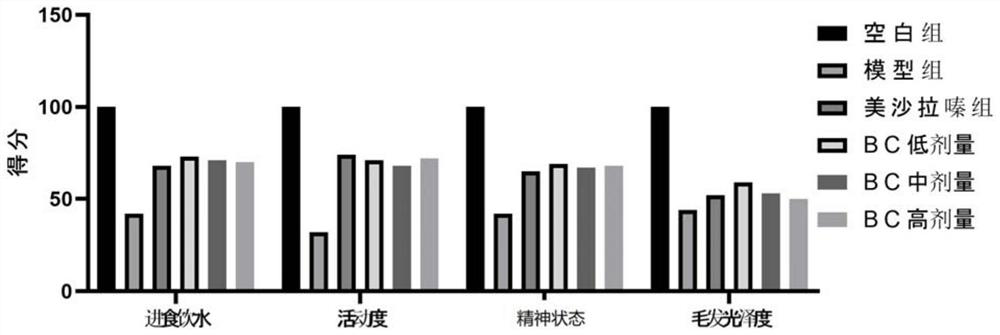
[0059] Example 2 The efficacy experiment of Bacteroides cellulolyticum in the treatment of Crohn's disease in mice induced by trinitrobenzenesulfonic acid (TNBS) combined with ethanol
[0060] experimental design
[0061] 60 SPF healthy BALB / c mice, female, average weight 18-22g, average age 6-8 weeks. After 1 week of adaptive feeding, they were randomly divided into groups as follows: blank group, model group, mesalazine group, low-, medium-, and high-dose groups of Bacteroides cellulolyticum (BC).
[0062] Modeling: In addition to the blank group, 2.0 mL of 3% chloral hydrate was injected intraperitoneally once a week, and after anesthesia, 0.1 mL of 2.0 mg TNBS / 50% ethanol mixed enema solution was administered as an enema using a 12 mm size mouse enema. The blank group was given 0.1 mL of normal saline by enema once a week. Administration for 6 consecutive weeks.
[0063] Dosing: After 6 weeks of modeling, except for the model group, the mice in each group were given the...
Embodiment 3
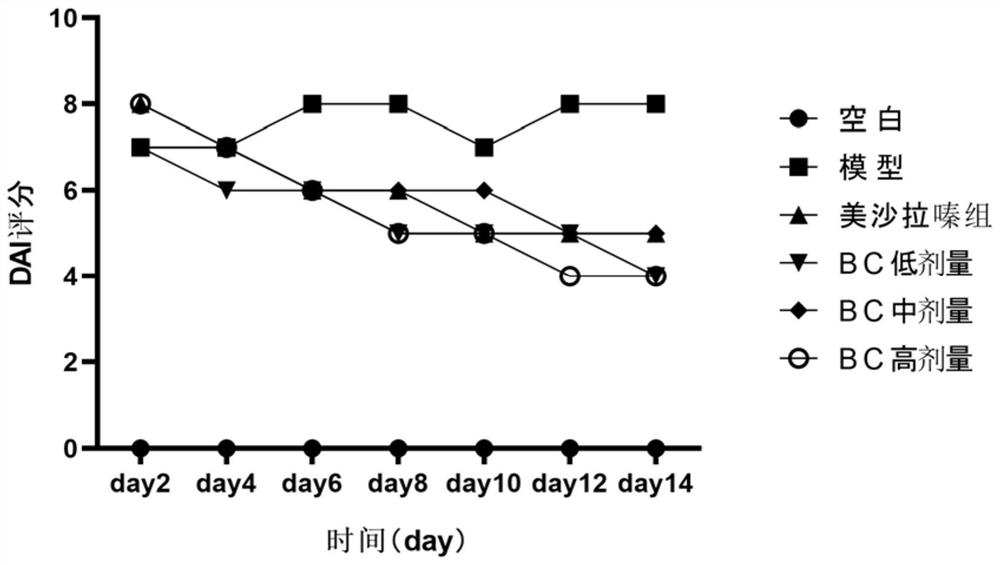
[0078] Example 3 Drug efficacy experiment of Bacteroides cellulolyticum in treating chronic ulcerative colitis in rats induced by dextran sulfate sodium salt (DSS)
[0079] experimental design:
[0080] Select 60 healthy and mature SD rats, half male and half female, body weight 200±20g, purchased from Guangdong Experimental Animal Center, raised in SPF grade animal room, randomly divided into 6 groups, 10 rats in each group, all rats before experiment Adapt to the environment for 1 week, and feed normal rat chow.
[0081] Using 5% dextran sulfate sodium salt (dextran sulfate sodium, DSS), purchased from Sigma, USA) solution to induce chronic ulcerative colitis model in rats, 60 SD rats were randomly divided into the following 6 groups:
[0082] Normal control group, model group, mesalazine group, BC low-dose group, BC medium-dose group, BC high-dose group, DAI score and tissue injury score were used to detect the curative effect of each intervention group. The animal groupi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com