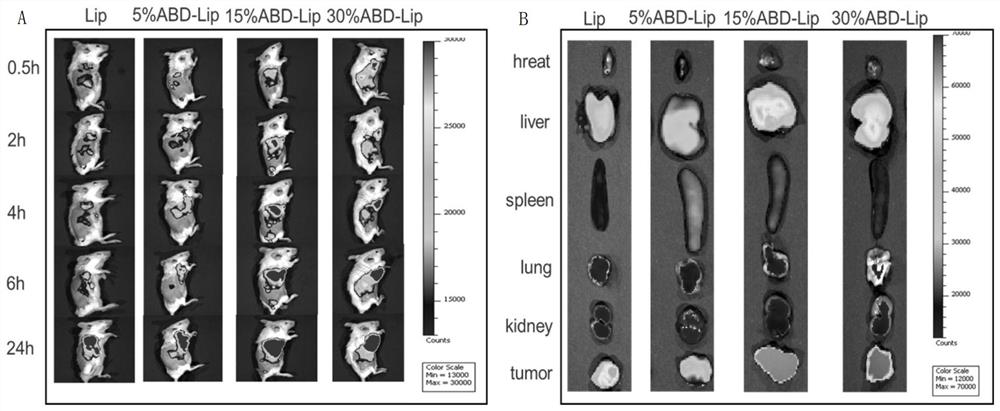
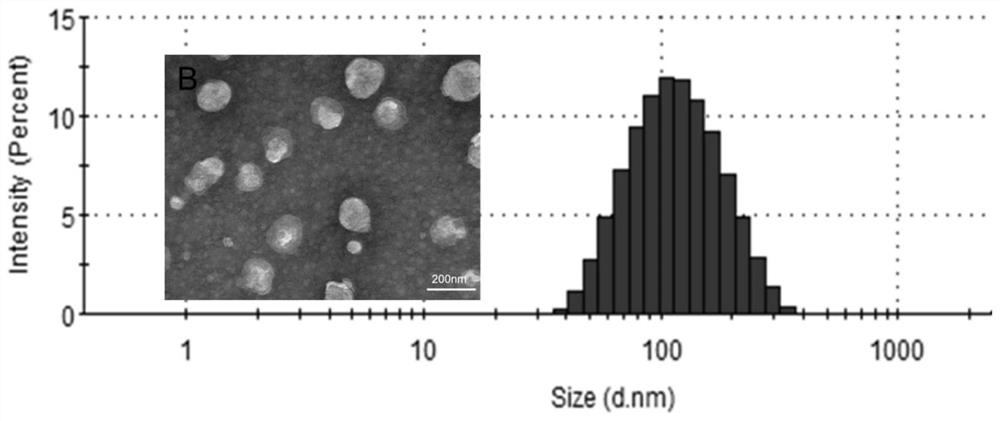
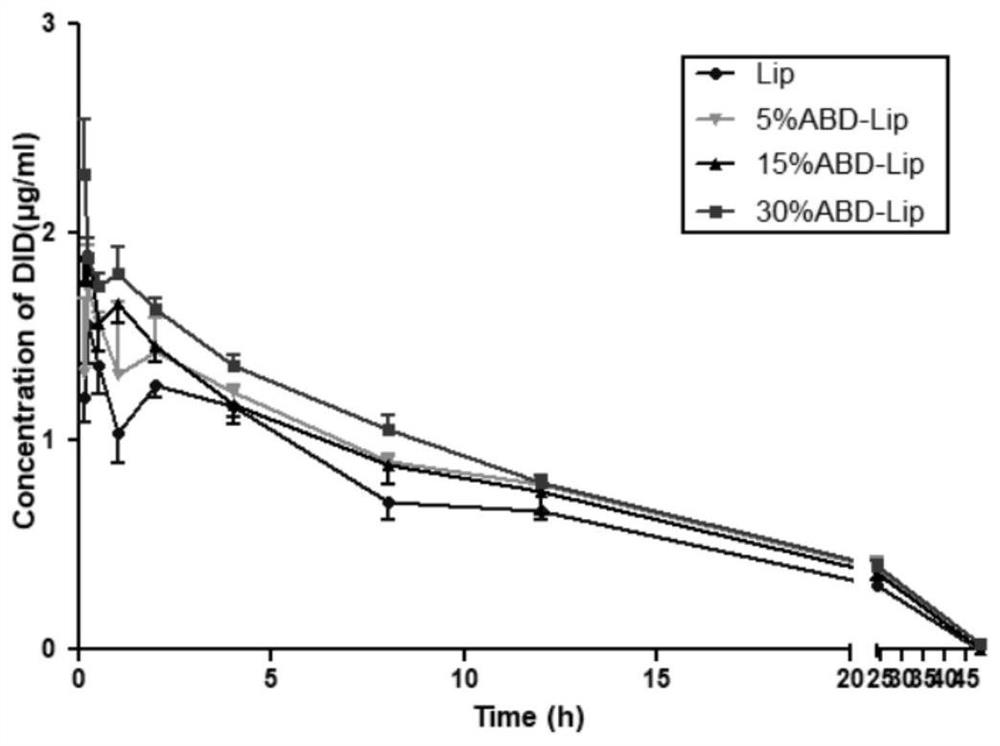
Doxorubicin long-circulating liposome targeted drug and preparation method thereof
A long-circulation liposome and doxorubicin technology, applied in the field of pharmacy, can solve the problems of short blood circulation time, poor solubility, unsatisfactory chemotherapy effect, etc., to achieve targeting and long circulation, easy to operate, cell less toxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] 1. Raw material prescription
[0069] type Features Dosage Proportion Soy Lecithin Liposome material 8.3mg 37.5% cholesterol Liposome material 2.6mg 11.8% DSPE-PEG2000 long-circulating liposome material 2.8mg 12.7% DSPE-PEG2000-MAL Liposome material for attachment of peptides 1.2mg 5.4% Adriamycin active ingredient 1.2mg 5.4% ABD peptide Bind specifically to albumin in vivo 6.0mg 27.2%
[0070] 2. Preparation process
[0071] (1) Preparation of PEGylated liposomes
[0072] Take the prescribed amount of 8.3mg soybean lecithin (S100), 2.6mg cholesterol (Chol), 2.8mg distearoylphosphatidylethanolamine-polyethylene glycol 2000 (DSPE-PEG2000), 1.2mg distearoylphosphatidylethanolamine- Polyethylene glycol 2000-maleimide (DSPE-PEG2000-MAL), the above lipid material was dissolved in 15mL of dichloromethane solvent, and the organic solvent was removed under reduced pressure at 30°C to obtain a lipid...
Embodiment 2
[0078] 1. Raw material prescription
[0079]
[0080]
[0081] 2. Preparation process
[0082] (1) Preparation of PEGylated liposomes
[0083] Take the prescription amount of 8.3mg soybean lecithin, 2.6mg cholesterol, 3.4mg DSPE-PEG2000, 0.6mg DSPE-PEG2000-MAL, dissolve the above lipid materials in 15mL of dichloromethane solvent, remove under reduced pressure at 30°C Organic solvents yield a lipid film.
[0084] (2) Preparation of drug-loaded liposomes
[0085] Hydrate the film with 123mM ammonium sulfate solution, then dialyze in a dialysis bag with a MW of 8K-14KDa for 1.5h, change the water every half an hour, then disperse the dialyzed solution with ultrasonic probe, and finally add 0.6mg of doxorubicin Incubate at 55°C for 10 minutes to obtain drug-loaded liposomes (DOX-Lip).
[0086] (3) Preparation of drug-loaded liposomes linked by ABD peptide
[0087] 2.3 mg of ABD peptide was dissolved in a small amount of HEPES buffer (pH=7.4), and stirred with the drug-...
Embodiment 3
[0089] 1. Raw material prescription
[0090] type Features Dosage Proportion Soy Lecithin Liposome material 8.3mg 45.6% cholesterol Liposome material 2.6mg 14.3% DSPE-PEG2000 long-circulating liposome material 3.8mg 20.9% DSPE-PEG2000-MAL Liposome material for attachment of peptides 0.2mg 1.1% Adriamycin active ingredient 1.8mg 9.9% ABD peptide Bind specifically to albumin in vivo 1.5mg 8.2%
[0091] 2. Preparation process
[0092] Same as Example 1 or 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com