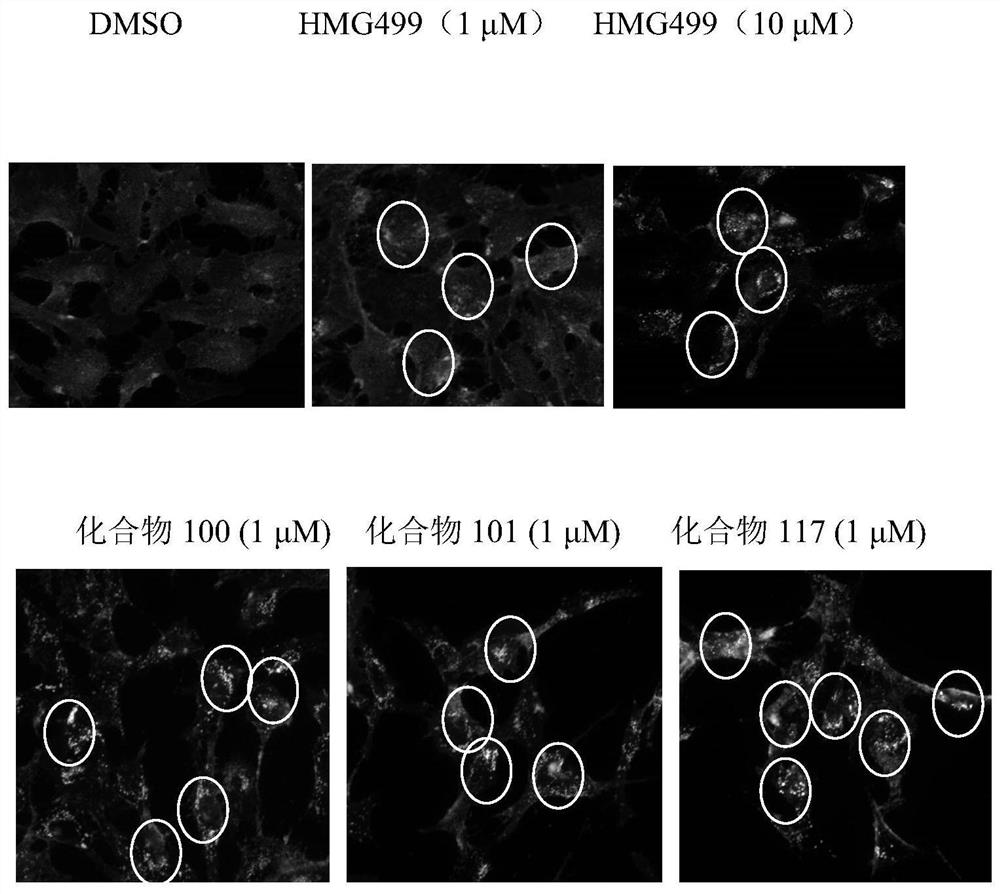
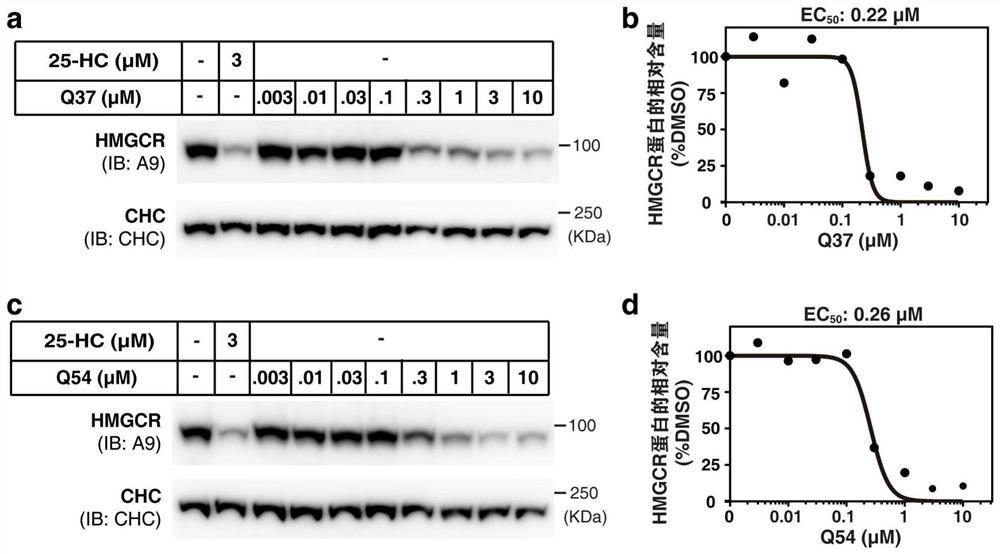
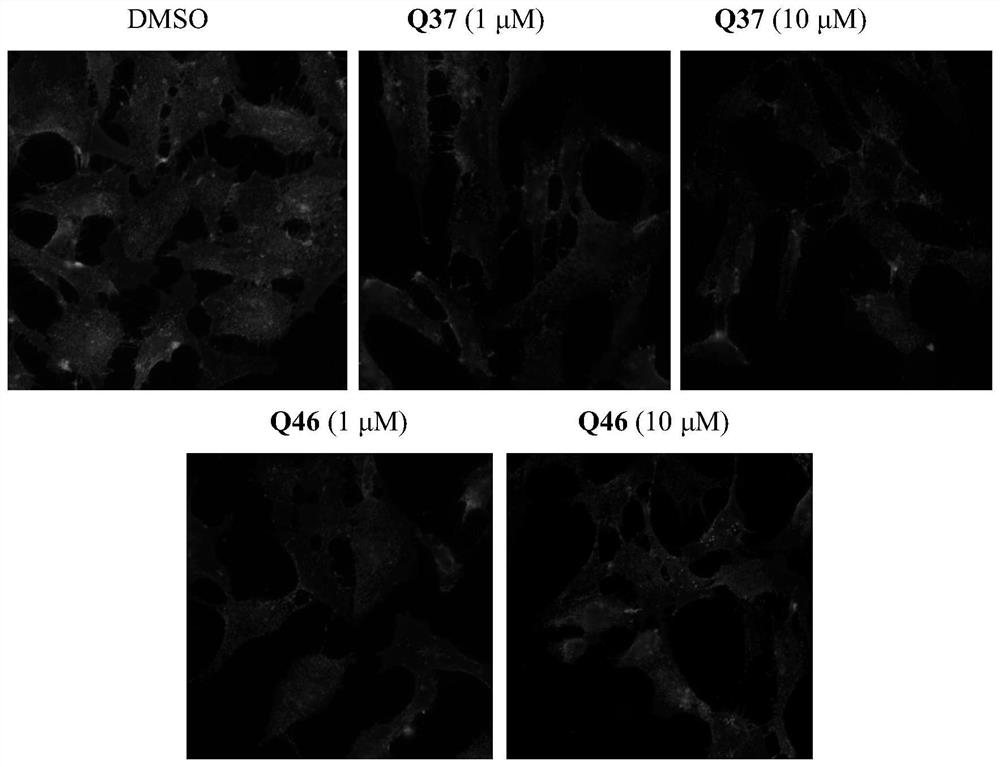
Cholic acid derivative and application thereof in lowering cholesterol
A derivative, cholic acid technology, applied in the field of medicine and its preparation and application, achieves good application prospects, reduces the increase of HMGCR protein, and reduces the effect of endogenous cholesterol levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0445] The preparation of embodiment 1 compound Q2-Q4
[0446] Compound Q1 (10g, 0.03mol) was placed in a 250mL single-necked flask, 150mL of anhydrous DMF was added and stirred until it dissolved, and imidazole (16.34g, 0.24mol), TBSCl (18.09g, 0.12mol), N 2 Replaced three times, stirred evenly and raised to room temperature, stirred for 5 hours, after TLC detected that the raw materials had reacted completely, added 50mL saturated ammonium chloride solution to quench the reaction, added ethyl acetate (100mL) for extraction and separation, and then used ethyl acetate for the aqueous phase (30mL×3) for extraction, and the organic phases were combined. The organic phase was washed with saturated ammonium chloride solution (50mL×2), water (50mL×3), saturated sodium chloride solution (50mL×2), and anhydrous Na 2 SO 4 It was dried and concentrated under reduced pressure to obtain compound Q2 (white solid 13.45 g, yield 99%), which was directly used in the next step.
[0447] Pu...
Embodiment 2
[0449] The preparation of embodiment 2 compound Q5
[0450] Compound Q4 (6.88g, 0.019mol), PDC (10.84g, 0.029mol), and silica gel (10.84g, 0.029mol) were placed in a 250mL single-necked flask, 100mL of anhydrous dichloromethane was added, stirred at room temperature for 8 hours, and detected by TLC The reaction of the raw materials is complete, remove the silica gel by suction filtration, wash the filter cake with dichloromethane (20mL×3), add saturated sodium sulfite to the system to quench for ten minutes until the system turns light green, extract and separate the liquid, and use dichloromethane (20mL×3) for the aqueous phase 3) extraction, combined organic phase. The organic phase was washed with water (30mL×3), saturated sodium chloride solution (30mL×2), and anhydrous Na 2 SO 4 After drying and concentration under reduced pressure, silica gel column chromatography (PE:EA=15:1) gave compound Q5 (6.83 g of white solid, yield 99%). 13 C NMR(101MHz,CDCl3)δ216.74,205.03,14...
Embodiment 3
[0451] The preparation of embodiment 3 compound Q6
[0452] Add NaH (3.42g, 0.142mol) in 250mL three-necked flask under ice-cooling, N 2 Replace three times, add 50 mL of anhydrous THF, stir to dissolve, slowly drop triethyl phosphoacetate (18.85 mL, 0.095 mol), drop it in about 10 minutes, and it can be seen that the system generates bubbles. The system was raised to room temperature and stirred for 10 minutes. Compound Q5 (6.83g, 0.019mol) was dissolved in anhydrous THF (50mL), dropped into the aforementioned system, stirred at room temperature for 1 hour, TLC detected that the reaction of the raw materials was complete, adding 50mL of saturated ammonium chloride solution to quench for ten minutes, and adding 60mL of acetic acid Ethyl extraction and liquid separation were performed, and the aqueous phase was extracted with ethyl acetate (20 mL×3), and the organic phases were combined. The organic phase was washed with saturated ammonium chloride (30mL×2), water (30mL×3), s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com