Heat-resistant beta-galactosidase and application thereof to lactose degradation
A technology of galactosidase and galactose, which is applied in the field of heat-resistant β-galactosidase and its application in lactose degradation, can solve the problems of poor heat resistance and low yield, and achieve simple purification methods and excellent physical and chemical properties , the effect of wide temperature stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Sequence analysis and recombinant expression of β-galactosidase Gal-T
[0024] The enzyme-producing gene Gal-T of the β-galactosidase Gal-T in the present invention is derived from the marine bacterium Baciliiussp.BY57, which contains a sequence of 3750 bases and encodes a sequence of 1250 amino acids. Using the conserved domain analysis of the National Center for Biotechnology Information (NCBI) to analyze the Conserved domain (CDD) and the multiple sequence alignment Basic Local Alignment Search Tool (Blast), it was found that the sequence contained a β-galactoside of the polysaccharide hydrolase GH family Enzyme conserved region. Among the reported β-galactosidases, the one with the highest amino acid sequence similarity to Gal-T is the β-galactosidase (Genbank AJ316559.1) of the polysaccharide hydrolase family 42 (GH42). The amino acid sequence similarity (Identity) is 75.75%. The β-galactosidase Gal-T described in this invention belongs to the polysacch...
Embodiment 2
[0033] Preparation and purification method of embodiment 2β-galactosidase Gal-T
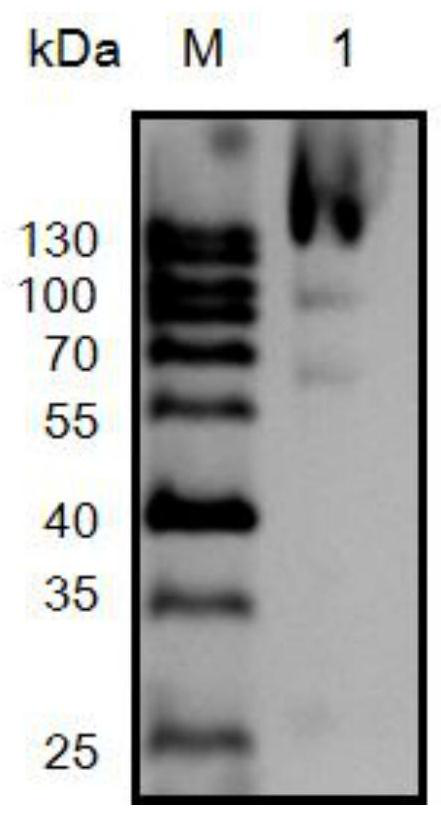
[0034] Culture the recombinant strain BL21(DE3) / pET20b-Gal-T in 100 mL of LB liquid medium (100 μg / mL ampicillin) in a shaker at 37°C at 200 / min to OD 600 =0.6, add the inducer isopropyl-β-D-thiogalactoside (IPTG) at a final concentration of 0.1 mM, and induce at 20° C. for 24 hours. The method for measuring the activity of β-galactosidase is: add 450 μL 10 mM o-nitrophenol-β-D-galactoside (ONPG) substrate (20 mM phosphate buffer, pH=8.0) to 50 μL enzyme solution, at 40 ° C After reacting for 10 min, add 500 μL of Na 2 CO 3 The reagent terminates the reaction. The mixture was centrifuged at 10,000 rpm for 10 min, and its absorbance was detected at OD420. Enzyme activity is defined as 1 U is the amount of enzyme required to produce 1 μM ONP per min. After testing, the chitosanase activity in the fermentation broth can reach 460.4U / mL.
[0035] After the fermentation stopped, centrifuge at 12...
Embodiment 3
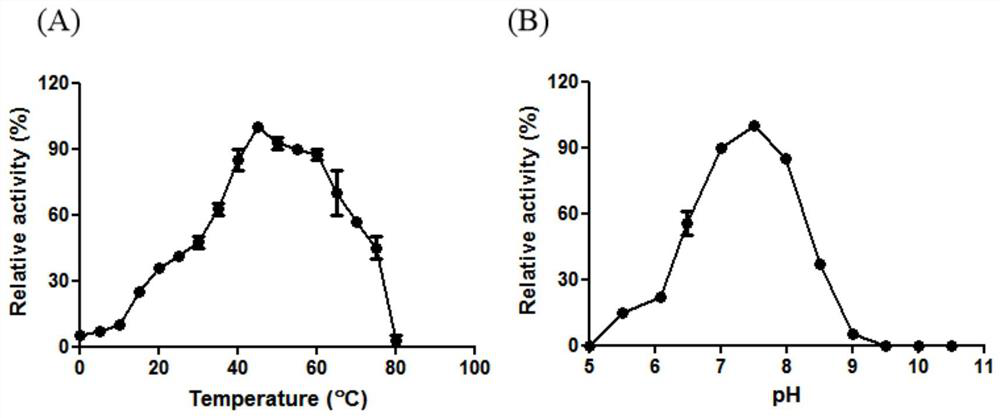
[0036] Optimum temperature and pH determination of embodiment 3β-galactosidase Gal-T
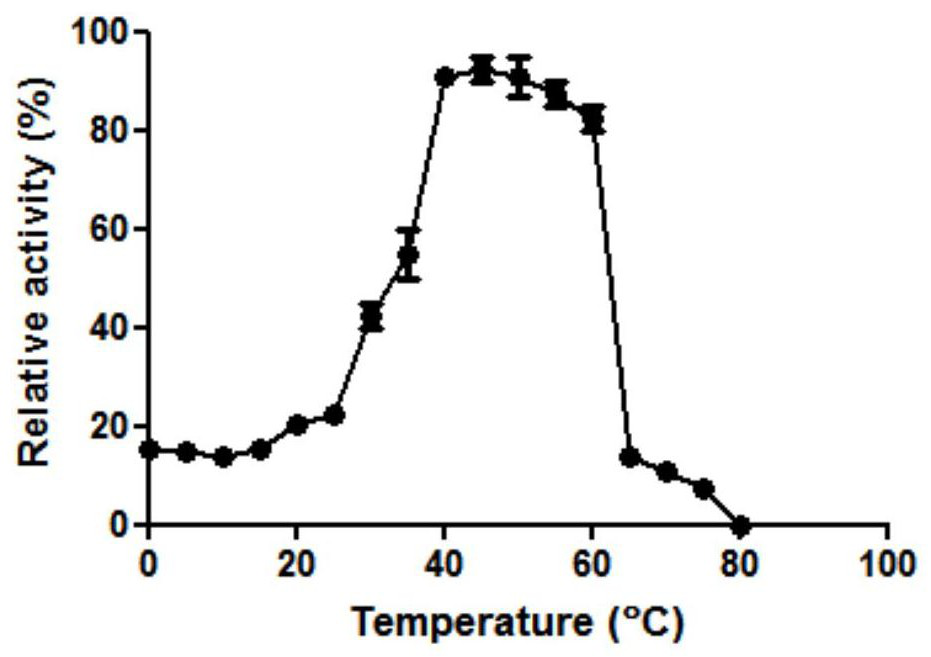
[0037] The β-galactosidase Gal-T purified in Example 2 was tested for enzyme activity under different conditions to detect the effects of different temperatures and pH on the enzyme activity. React at different temperatures (0-80° C.) for 10 min, detect the effect of different reaction temperatures on enzyme activity, and calculate the relative enzyme activity of Gal-T at different temperatures with the highest enzyme activity as 100%. like figure 2 As shown in A, the optimal reaction temperature of β-galactosidase Gal-T is 45°C.
[0038] The β-galactosidase Gal-T purified in Example 2 was reacted with the lactose substrate of the Britton-Robinson buffer system (PH5.5-10.5). The buffer consists of phosphoric, boric and acetic acids, to which different amounts of sodium hydroxide can be added to form a buffered solution with a wide pH range. The activity was detected at the optimum temper...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com