Method for preparing lithium manganate positive electrode material from pyrolusite
A cathode material and pyrolusite technology, which is applied in the field of pyrolusite to prepare lithium manganate cathode material, can solve the problems of single source of raw materials and low economic value, and achieve the effects of single source of raw materials, less impurity content and abundant raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
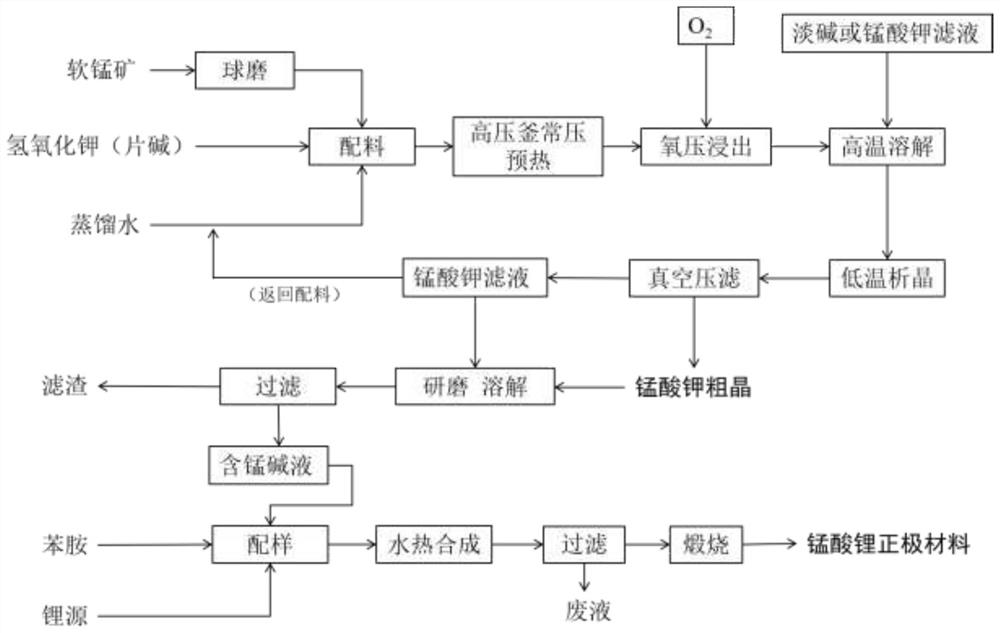
[0060] (1) Use a planetary ball mill to crush and grind pyrolusite at high speed to obtain manganese ore with a particle size of about 66um.
[0061] (2) 70g of pyrolusite and 537.4mL of 65% potassium hydroxide solution were placed in an autoclave reactor for pressurized alkaline leaching, stirred at a stirring speed of 500r / min, the leaching temperature was 260°C, and the oxygen pressure was controlled at 0.3Mpa. The leaching time is 150min;
[0062] After oxygenation, redox reaction occurs; take out the reacted slurry after depressurization, dissolve it at high temperature with 2mol / L potassium oxide solution, then slowly cool and crystallize. Filtration, the filter cake is potassium manganate coarse crystals.
[0063] (3) Dissolving, grinding, and filtering the coarse crystals of potassium manganate obtained in the step (2) and a certain amount of filtrate. Weigh the coarse crystals of potassium manganate and potassium manganate filtrate for grinding and dissolving. The g...
Embodiment 2
[0068] (1) Use a planetary ball mill to crush and grind pyrolusite at high speed to obtain manganese ore with a particle size of about 66um.
[0069] (2) 70g of pyrolusite and 537.4mL of 65% potassium hydroxide solution were placed in an autoclave reactor for high-pressure alkaline leaching, stirred at a stirring speed of 500r / min, the leaching temperature was 250°C, and the oxygen pressure was controlled at 0.5Mpa. The time is 180min.
[0070] After passing oxygen, redox reaction occurs; take out the reacted slurry after depressurization, dissolve it at high temperature with 2mol / L potassium oxide solution or electrolytic mother liquor, and then slowly cool and crystallize, and after reaching a certain degree of crystallization, put The crystallization liquid is filtered, and the filter cake is coarse crystals of potassium manganate.
[0071] (3) Dissolving, grinding, and filtering the coarse crystals of potassium manganate obtained in the step (2) and a certain amount of fi...
Embodiment 3X
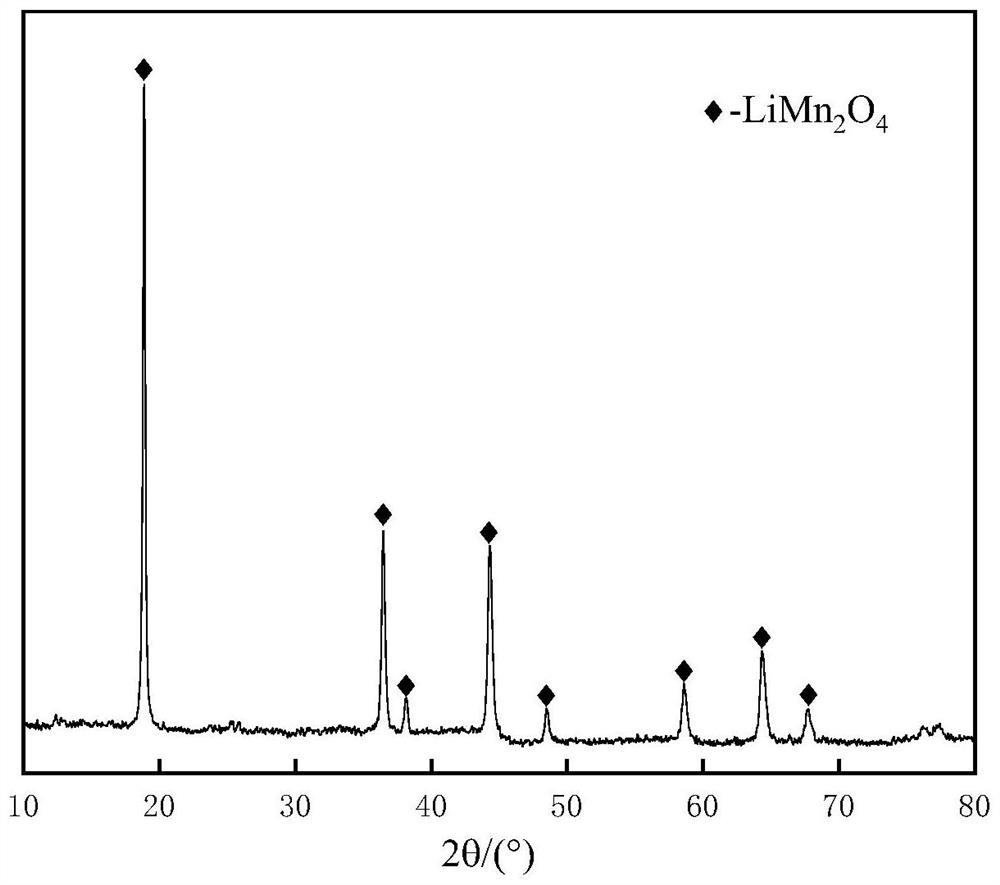
[0075] Example 3 X-ray Diffraction
[0076] The lithium manganate cathode material obtained in Example 1 was subjected to X-ray diffraction, and the X-ray diffraction conditions were as follows: the model of the diffractometer was D / Max2500, the radiation source was Cu-Kα, the wavelength was 0.15406nm, and the scan rate was 5° min- 1 , the scanning range is from 5° to 90°, and the scanning diffraction pattern is as follows figure 2 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com