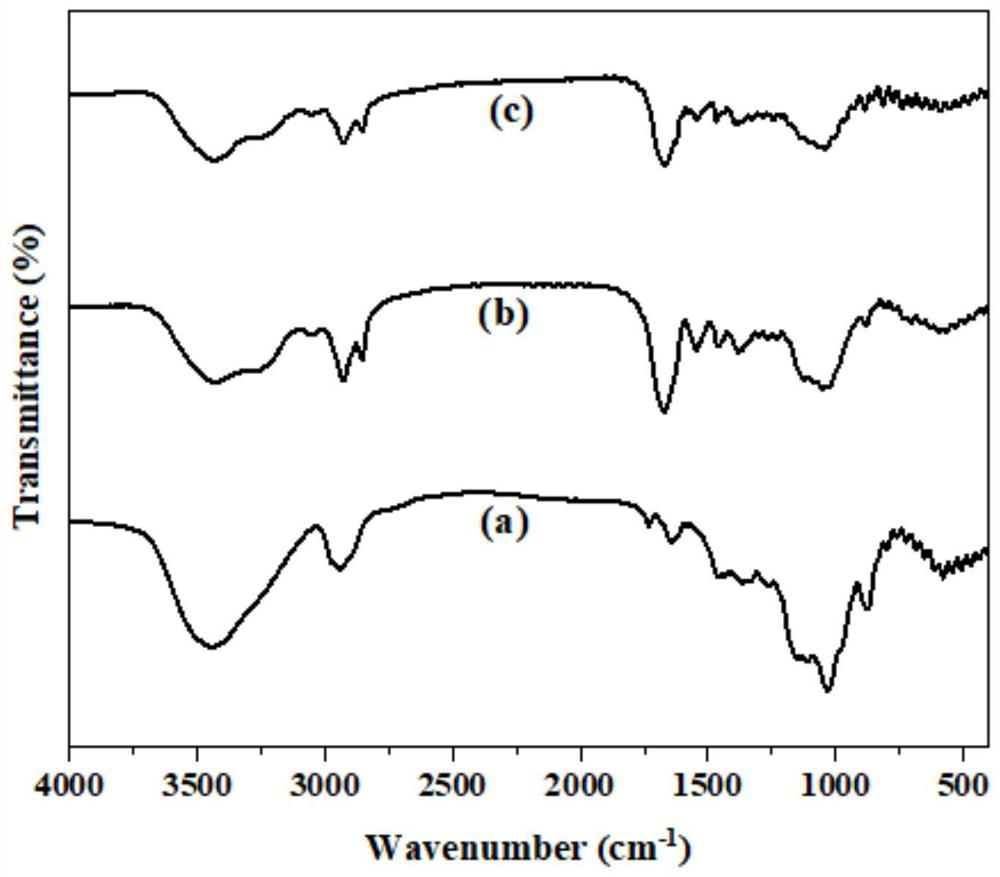
Dialdehyde cellulose-based Schiff base fluorescent probe for detecting Al <3+>, and preparation method and application thereof
A technology of dialdehyde cellulose and base Schiff bases, applied in the field of fluorescence detection, can solve the problems of difficult processing of fluorescent small molecules, cumbersome operation, low sensitivity, etc., achieve good application prospects, overcome limitations, and have good luminescent performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The synthetic reaction formula of dialdehyde cellulose-sebacylhydrazide-2-hydroxyl-1-naphthaldehyde bis-Schiff base is as follows:
[0030]
[0031] Add 1.0g dialdehyde cellulose, 50mL ethylene glycol monomethyl ether and 1.84g sebacic hydrazide to a 100mL three-necked flask equipped with a stirrer, thermometer and reflux condenser, and then add 3 to 5 drops of acetic acid , Stirring and reflux reaction at 125°C for 24h. The reaction solution was filtered by suction, washed with hot ethylene glycol monomethyl ether and distilled water, and dried in vacuum at 45°C for 24 hours to obtain dialdehyde cellulose-sebacylhydrazide mono-Schiff base. Add 0.5g of dialdehyde cellulose-sebacylhydrazide mono-Schiff base and 0.82g of 2-hydroxy-1-naphthaldehyde into 50mL of ethanol, drop 3-5 drops of acetic acid, and then stir and reflux at 80°C for 24h , the reaction solution was filtered by suction, washed with ethanol, and dried in vacuum at 45°C for 24 hours to obtain bis-Schif...
Embodiment 2
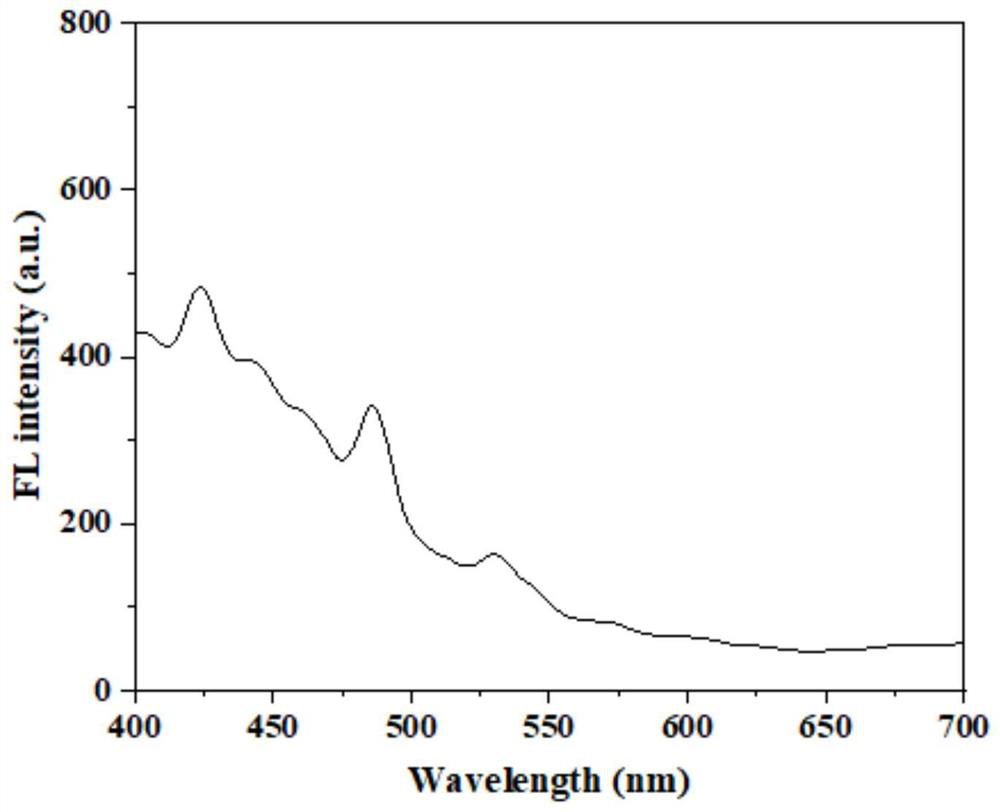
[0034] The prepared bisaldehyde cellulose-sebacylhydrazide-2-hydroxyl-1-naphthaldehyde bis-Schiff base was pressed into tablets, and its solid-state fluorescence emission spectrum was measured, as shown in figure 2 shown. The results show that the maximum emission wavelength of solid fluorescence is at 425nm.
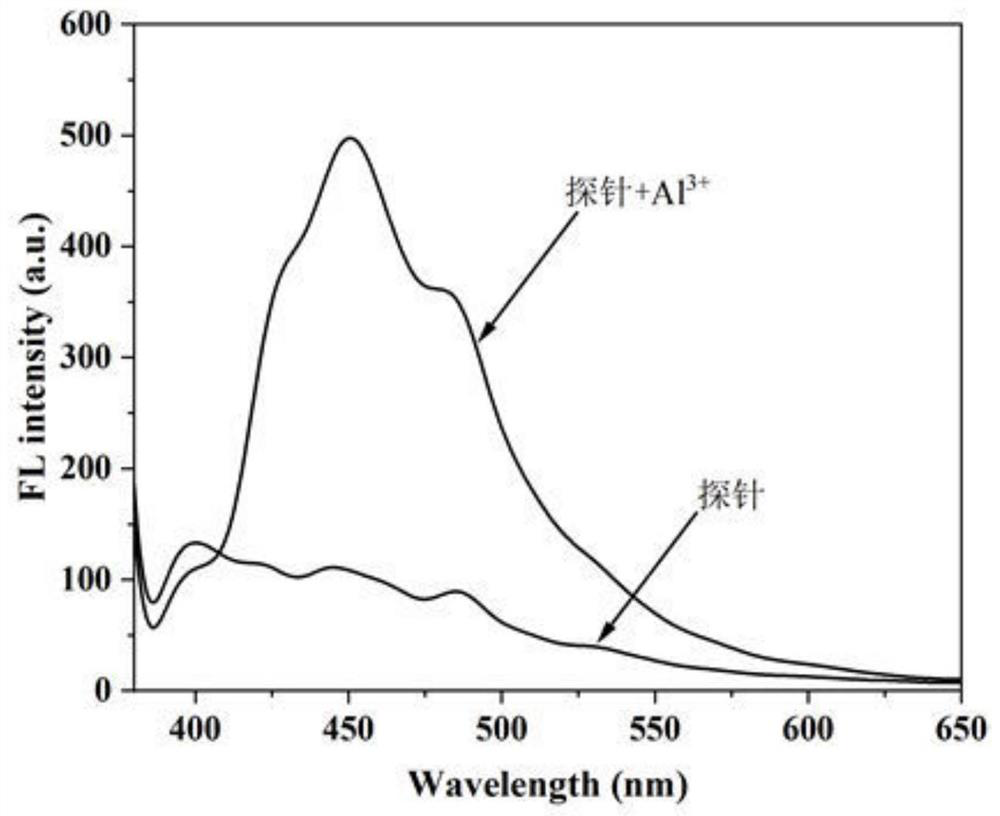
[0035] Dialdehyde cellulose-sebacylhydrazide-2-hydroxyl-1-naphthaldehyde bis-Schiff base was added to DMF to form a DMF suspension with a concentration of 8.0 mg / mL, and the fluorescence emission spectrum in DMF was measured. like image 3 shown. The results show that in the DMF system, without adding Al 3+ When the fluorescence intensity is weak, when Al is added 3+ Afterwards, the fluorescence intensity increased sharply, and the maximum emission wavelength was 450nm (the excitation wavelength was 355nm, the excitation slit broadband was 15nm, and the emission slit broadband was 5.0nm).
[0036] Add dialdehyde cellulose-sebacylhydrazide-2-hydroxyl-1-naphthaldehy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com