Naringenin in-vitro enzymatic synthesis method based on malonyl coenzyme A regeneration
The technology of a malonyl coenzyme and a synthesis method is applied in the field of in vitro enzymatic synthesis of naringenin to achieve the effects of simple product separation, simple composition and easy reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
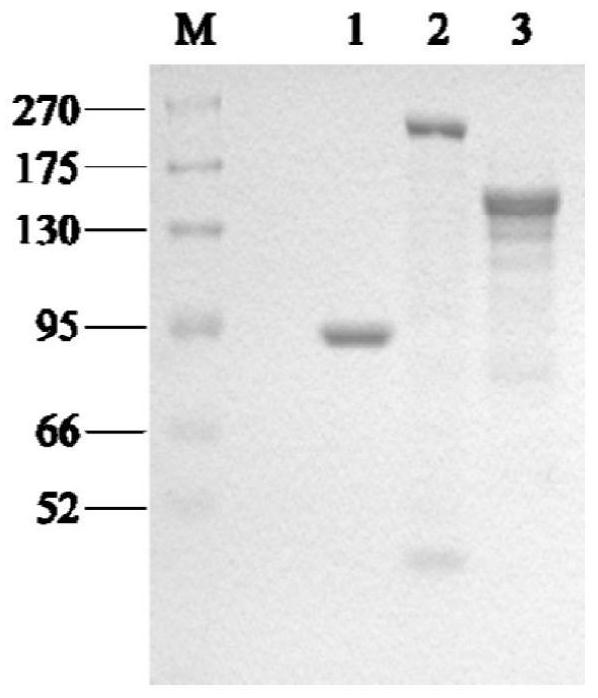
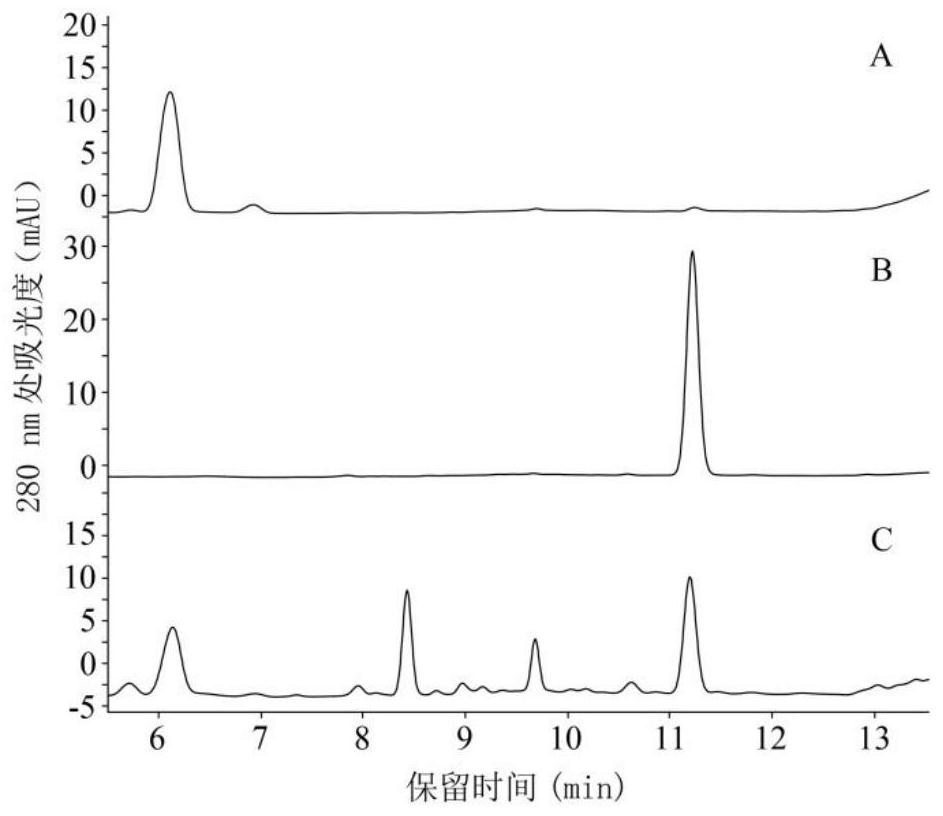
Image
Examples
Embodiment 1
[0042] 1. Construction of recombinant plasmids
[0043] Escherichia coli acetyl-CoA synthetase gene provided by GenBank database ACS and yeast acetyl-CoA carboxylase gene ACC1 nucleotide sequence information, design two pairs of primer sequences for the ACS The coding region of the gene was cloned into the Escherichia coli expression vector pET-32a(+), and the ACC1 The coding region of the gene was cloned into the Pichia pastoris expression vector pGAP-Neo:
[0044] PCR amplification ACS The forward primer of the gene is 5'-GCTGATATCGGATCC GAATTC atgagccaaattcacaaacac-3' (as shown in SEQ ID No.3), the base in italics indicates the enzyme cutting site EcoRI; the reverse primer is 5'-GTGGTGGTGGTGGTG CTCGAG ttacgatggcatcgcgatag-3' (as shown in SEQ ID No.4), the base in italics indicates the restriction site XhoI.
[0045] PCR amplification ACC1 The forward primer of the gene is
[0046] 5'-CTATTTCGAAACGAG GAATTC ATGAGCGAAGAAAGCTTATTC-3' (as shown in SEQ ID No.5), t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com