Synthesis method of 3-(3-hydroxyphenyl)-1, 1-dimethylurea, intermediate and application
A technology of dimethylurea and hydroxyphenyl, which is applied in the field of drug synthesis, can solve the problems of drug quality control risks, cumbersome operations, and residues, and achieve the effects of improving reaction processing efficiency, simple operation process, and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
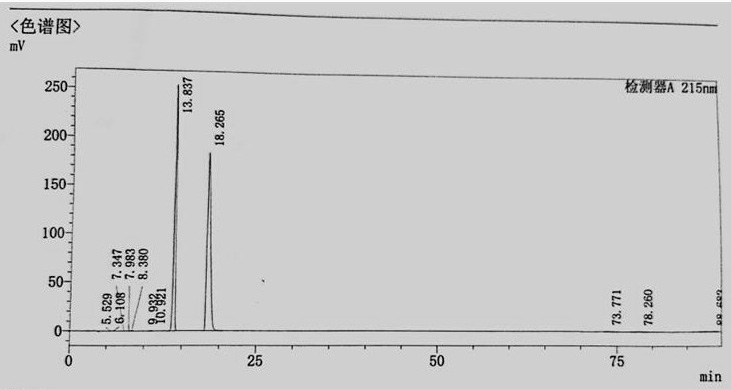
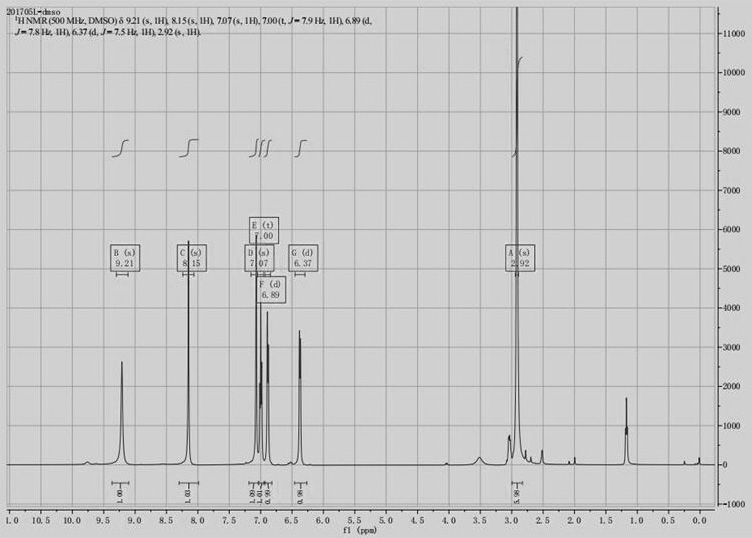
Image
Examples
Embodiment 1
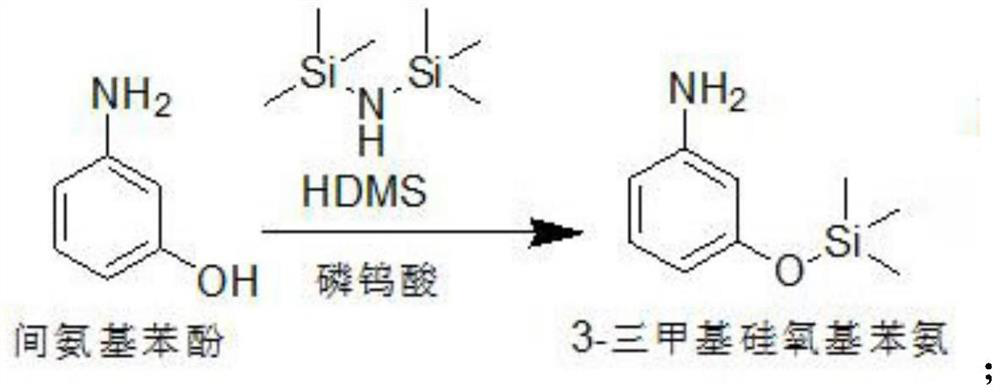
[0030] A kind of synthetic method of 3-(3-hydroxyphenyl)-1,1-dimethylurea, comprises the following concrete steps:
[0031] S1. Preparation of intermediate compound Ⅰ: Add raw materials m-aminophenol 20g (0.183mol), ethyl acetate 60ml, HDMS (hexamethylsilazane) 32g (0.198mol, 1.08eq) and phosphotungstic acid into a single-necked round bottom flask 0.5g (0.17mmol), heat up to 55-70°C with stirring, keep stirring for 2h, after the reaction, cool down to room temperature, add 100g tap water to wash until pH = 7.0, take the organic phase, add magnesium sulfate for dehydration, and obtain 3- The ethyl acetate solution of trimethylsiloxyaniline, reaction equation is:
[0032]
[0033] S2. Preparation of intermediate compound II: add 19 g of triethylamine (0.187 mol, 1.03 eq) to the ethyl acetate solution of 3-trimethylsiloxyaniline after dehydration, and start to drop 20 g of dimethylcarbamoyl chloride (0.186mol, 1.01eq), heated to 55-70°C, kept stirring for 6h, filtered to remo...
Embodiment 2
[0038] A kind of synthetic method of 3-(3-hydroxyphenyl)-1,1-dimethylurea, comprises the following specific steps:
[0039] S1. Preparation of intermediate compound I: Add 10g (0.092mol) of raw materials m-aminophenol, 40ml of toluene, 15.9g (0.10mol, 1.08eq) of HDMS (hexamethylsilylazide) and 0.26 phosphotungstic acid into a single-necked round bottom flask g (0.08mmol), heated to 55-70°C under stirring, kept stirring for 1.5h, after the reaction, cooled to room temperature, added 70g tap water to wash to PH=7.0, took the organic phase, added magnesium sulfate for dehydration, and obtained 3- The toluene solution of trimethylsiloxyaniline;
[0040] S2. Preparation of intermediate compound II: Add 10.1 g (0.10 mol, 1.09 eq) of triethylamine to the toluene solution of 3-trimethylsiloxyaniline after dehydration, and start to drop 10.8 g of dimethylcarbamoyl chloride (0.10mol, 1.09eq), heated to 55-70°C, kept stirring for 6h, filtered to remove the solid, and washed the organic ...
Embodiment 3
[0043] A kind of synthetic method of 3-(3-hydroxyphenyl)-1,1-dimethylurea, comprises the following concrete steps:
[0044] S1. Preparation of intermediate compound I: Add 30g (0.274mol) of raw materials m-aminophenol, 150ml of acetone, 50g (0.31mol, 1.13eq) of HDMS (hexamethylsilylazide) and 3g of phosphotungstic acid ( 0.001mol), heated to 55-70°C with stirring, kept stirring for 1h, after the reaction was completed, cooled to room temperature, added 75g tap water to wash until PH = 7.0, took the organic phase, added magnesium sulfate for dehydration, and obtained 3-trimethyl Acetone solution of siloxyaniline;
[0045] S2. Preparation of intermediate compound II: Add 35 g of triethylamine (0.346 mol, 1.26 eq) to the acetone solution of dehydrated 3-trimethylsilyloxyaniline, and start to drop 36 g of dimethylcarbamoyl chloride ( 0.335mol, 1.22eq), heated to 55~70℃, kept stirring for 6h, filtered to remove the solid, washed the organic phase twice with 350g water to obtain 1,1,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com