Gonadorelin for injection for veterinary use and preparation method of gonadorelin for injection
A technology of freeze-dried powder injection and chorionic gonadotropin, which is applied in freeze-dried delivery, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. Collapse, chorionic gonadotropin damage, etc., to prevent the destruction of the vitrified state, improve the stability of the drug, and ensure the quality of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] Wherein, the concentration of the gelatin solution is 4%, and the coagulation agent is a sodium sulfate solution with a concentration of 60%.
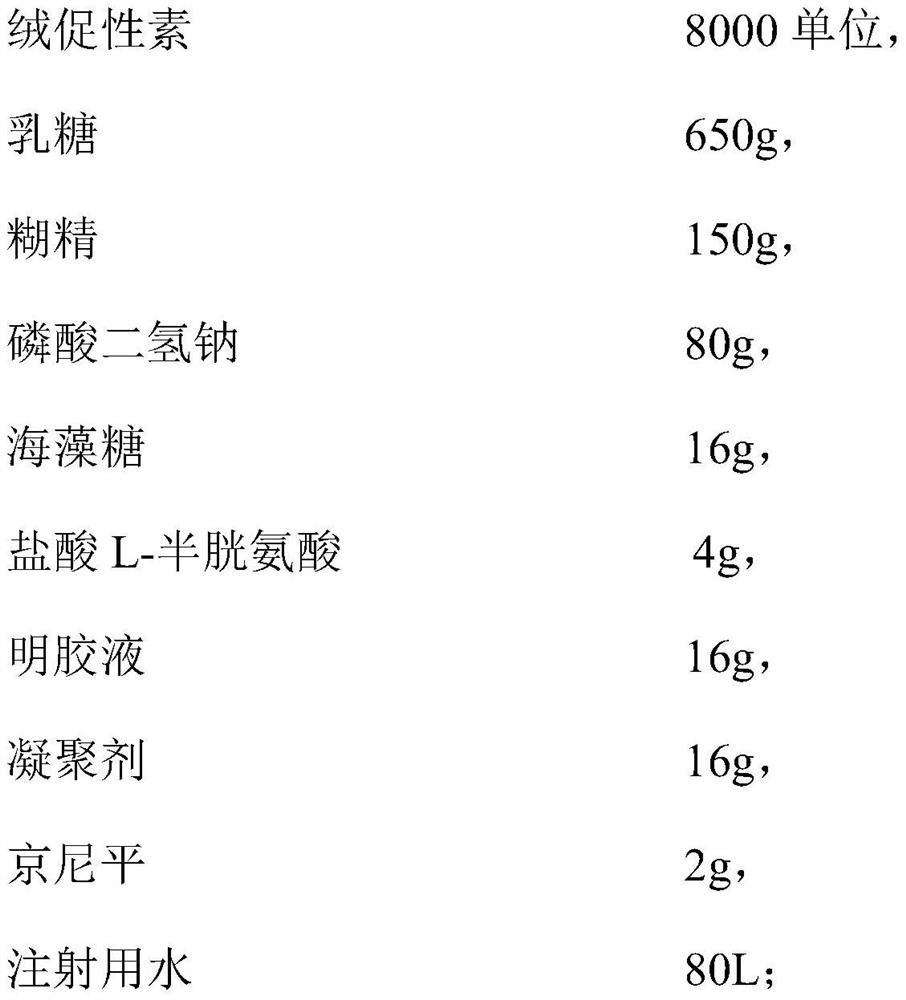
[0037] (1) Add 8000 units of chorionic gonadotropin, 650g lactose, 150g dextrin, 16g trehalose, 80g sodium dihydrogen phosphate, 4g L-cysteine hydrochloride to the water for injection, and process through macroporous adsorption resin after dissolving, Add 16g of gelatin solution and 16g of coagulant, and then stir at 350rpm for 30 minutes; adjust the pH to 4.5-6.5 with a pH regulator, add 2g of genipin, continue to stir, and then filter and sterilize through a 0.22μm sterile filter to prepare into microcapsule liquid;
[0038] (2) Reduce the temperature of the condenser of the lyophilizer to below -45°C in advance; the liquid medicine is filled into ampoules under the protection of nitrogen, and fed to the lyophilizer;
[0039] (3) Reduce the temperature of the plate layer to below -40°C within 1h, freeze for 2h; ...
Embodiment 2
[0047]
[0048] Wherein, the concentration of the gelatin solution is 4%, and the coagulation agent is a sodium sulfate solution with a concentration of 60%.
[0049](1) Add 8000 units of chorionic gonadotropin, 1000g lactose, 200g dextrin, 24g trehalose, 120g sodium dihydrogen phosphate, 6g L-cysteine hydrochloride to the water for injection, and process through macroporous adsorption resin after dissolving, Add 24g of gelatin solution and 8g of coagulant, and then stir at a speed of 350rpm for 30min; adjust the pH to 4.5-6.5 with a pH regulator, add 3g of genipin, continue to stir, and then filter and sterilize through a 0.22μm sterile filter to prepare into microcapsule liquid;
[0050] (2) Reduce the temperature of the condenser of the lyophilizer to below -45°C in advance; the liquid medicine is filled into ampoules under the protection of nitrogen, and fed to the lyophilizer;
[0051] (3) Reduce the temperature of the plate layer to below -40°C within 1h, freeze fo...
Embodiment 3
[0058]
[0059]
[0060] Wherein, the concentration of the gelatin solution is 4%, and the coagulation agent is a sodium sulfate solution with a concentration of 60%.
[0061] (1) Add 8000 units of chorionic gonadotropin, 1200g of lactose, 400g of dextrin, 32g of trehalose, 160g of sodium dihydrogen phosphate, 8g of L-cysteine hydrochloride to 80L of water for injection, and process it through macroporous adsorption resin after dissolving , add 48g of gelatin solution and 12g of coagulant, and then stir at a speed of 350rpm for 30min; adjust the pH to 4.5 to 6.5 with a pH regulator, add 4g of genipin, continue to stir, and then filter and sterilize through a 0.22μm sterile filter. Formulated into microcapsule liquid medicine;
[0062] (2) Reduce the temperature of the condenser of the lyophilizer to below -45°C in advance; the liquid medicine is filled into ampoules under the protection of nitrogen, and fed to the lyophilizer;
[0063] (3) Reduce the temperature of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com