Application of methoxy flavonoid compound in preparation of anti-vitiligo medicine
A technology of methoxyflavones and compounds is applied in the field of preparation of anti-vitiligo drugs, and can solve the problems of non-universal treatment methods, difficult clinical promotion of photochemotherapy, and death of melanocytes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] For details of experimental materials and animals, see the content of the appeal.
[0048] The name and treatment situation of each group in this embodiment are as follows: (1) blank control group (Ctrl); (2) methoxysalen positive drug group (8-MOP, 25 μ mol / L); (3) kaempferol group (2 -64 μmol / L); (4) isorhamnetin group (4-64 μmol / L). Place them in water at a temperature of 24-28°C for feeding, light for 14 hours a day, and place them in darkness for 10 hours. The specific treatment is as follows:
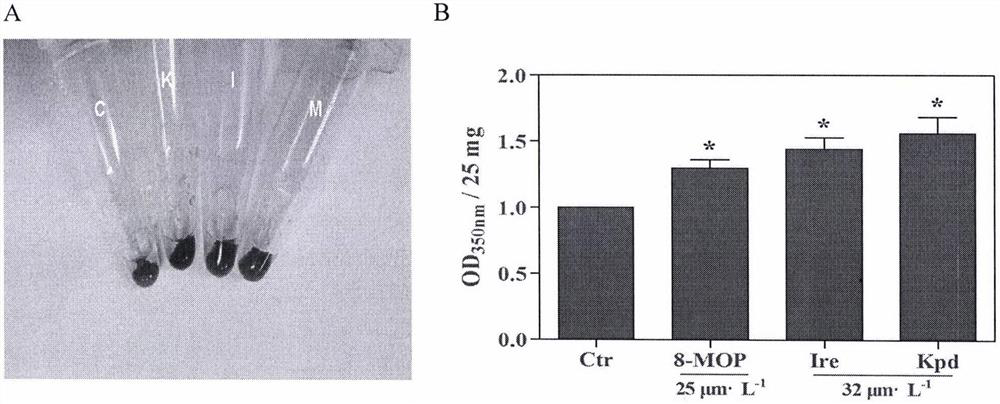
[0049] 100 adult zebrafish aged 4-12 months were randomly divided into 4 groups, 25 in each group. Control group: treated with 550nmol / L new copper reagent for 20 days, natural restoration of melanin regeneration for 3 days; positive control group: treated with 550nmol / L new copper reagent for 20 days, 25μmol / L methoxysalen (8-MOP) for 3 days Isorhamnetin group: treated with 550nmol / L new copper reagent for 20 days, treated with different concentrations of isorhamnetin (...
Embodiment 2
[0051] The name and treatment situation of each group in this embodiment are as follows: (1) blank control group (Ctrl); (2) methoxysalen positive drug group (8-MOP, 25 μ mol / L); (3) kaempferol group (2 -64 μmol / L); (4) isorhamnetin group (4-64 μmol / L). Place them in water at a temperature of 24-28°C for feeding, light for 14 hours a day, and place them in darkness for 10 hours. The specific treatment is as follows:
[0052] 100 adult zebrafish aged 4-12 months were randomly divided into 4 groups, 25 in each group. Control group: treated with 550nmol / L new copper reagent for 20 days, natural restoration of melanin regeneration for 3 days; positive control group: treated with 550nmol / L new copper reagent for 20 days, 25μmol / L methoxysalen (8-MOP) for 3 days Isorhamnetin group: treated with 550nmol / L new copper reagent for 20 days, treated with different concentrations of isorhamnetin (Ire) for 3 days; kaempferol group: treated with 550nmol / L new copper reagent for 20 days, us...
Embodiment 3
[0055] The name and processing situation of each group in this embodiment are as follows:
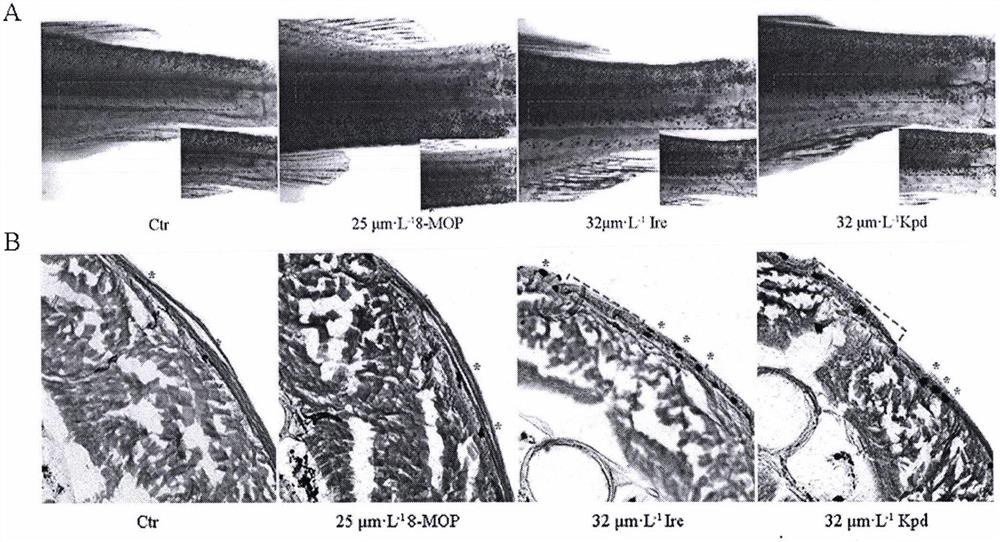
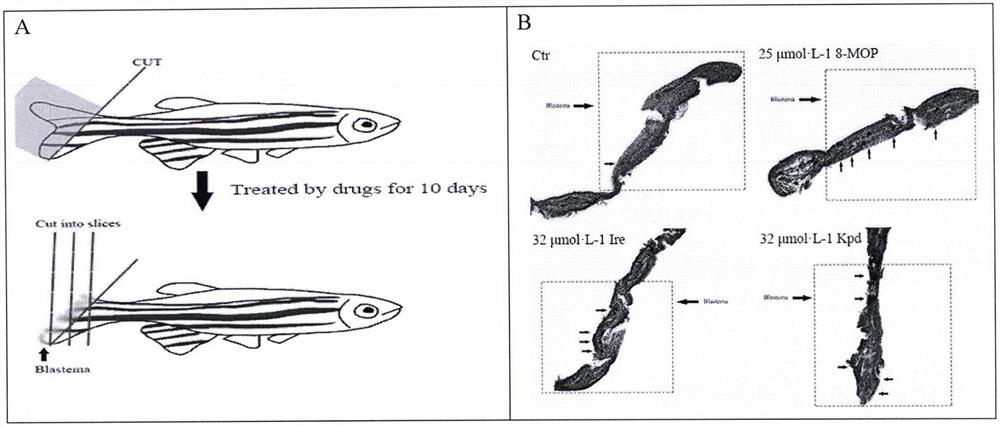
[0056] (1) blank control group (Ctrl); (2) methoxysalen positive drug group (8-MOP, 25 μmol / L); (3) kaempferol group (2-64 μmol / L); (4) isorhamna Plain group (4-64μmol / L). Place them in water at a temperature of 24-28°C for feeding, light for 14 hours a day, and place them in darkness for 10 hours. Zebrafish aged 4 to 12 months were taken, and their caudal fins were obliquely cut off. They were treated with kaempferol, isorhamnetin and methoxsalen respectively for 10 days, and the newborn primordia of the zebrafish tail were taken for horizontal frozen tissue sections, stained with hematoxylin. solution for staining, the results are as follows image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com