A Gas Diffusion Cathode and an Electrochemical Reactor for In Situ Hydrogen Peroxide Production
A gas diffusion cathode and reactor technology, which is applied in chemical instruments and methods, oxidized water/sewage treatment, electrodes, etc., can solve the problems of high cost, material toxicity, and rapid increase in the development of carbon-based materials, and achieve low cost and high efficiency. The effect of concentration, simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
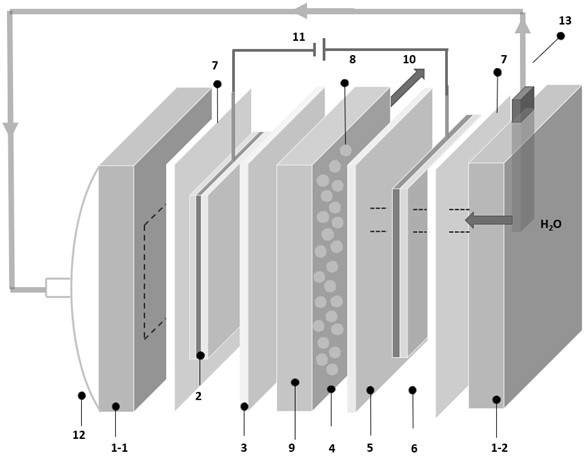
[0061] This embodiment discloses a gas diffusion cathode, the specific structure can be found in figure 1 As shown in the label 2, the gas diffusion cathode includes a diffusion layer, a current collector substrate and a catalytic layer arranged in sequence;
[0062] The base of the current collector is made of nickel foam, the nickel foam has a porosity of 95%, a pore diameter of 0.2-0.6mm, a thickness of 1.0mm, and a surface density of 325g / m 2 , its preparation method is:
[0063] Cut the nickel foam into a suitable shape, and use deionized water and ethanol to ultrasonically clean it for later use;
[0064] The diffusion layer is made of carbon black and PTFE emulsion in proportion, and the diffusion layer is coated on one side of the current collector substrate, and the preparation method is as follows:
[0065] (1) Mix carbon black particles and absolute ethanol at a ratio of 1g:20ml, and ultrasonically stir evenly; then add dropwise PTFE emulsion with a mass fraction...
Embodiment 2
[0077] This embodiment discloses an electrochemical reactor for producing hydrogen peroxide in situ, see Figure 1~4 As shown, the reactor includes a cathode end plate 1-1, a silica gel pad 7, a gas diffusion cathode 2 described in Example 1, an anion exchange membrane 3, a solid electrolyte layer 4, a cation exchange membrane 5, and a platinum anode arranged in sequence. 6. Silica gel pad 7, anode end plate 1-2, movable power supply 11 connected to both ends of gas diffusion cathode 2 and platinum anode 6, in this embodiment, the cathode end plate 1-1, anode end plate 1-2, The solid electrolyte layer 4 is made of organic glass plate.
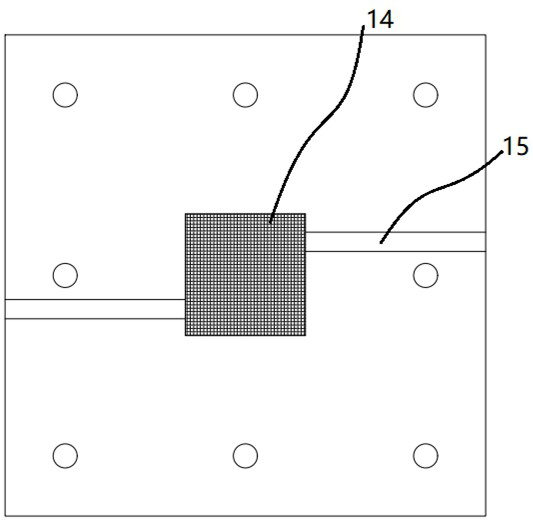
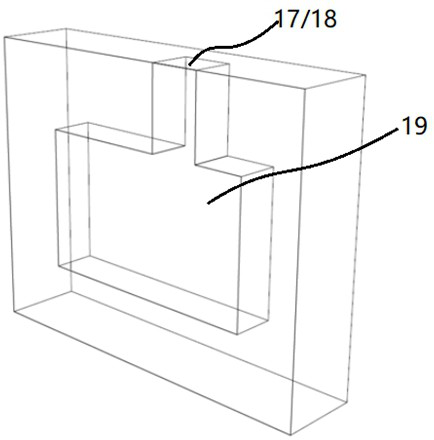
[0078] The center of the cathode end plate 1-1 is provided with an opening for air to pass through, and the anode end plate 1-2 is provided with an anode reaction chamber 19 communicating with the cation exchange membrane 3, and the anode end plate 1-2 A water inlet 17 and an air outlet 18 communicating with the anode reaction chamber 19 are p...
Embodiment 3
[0083] In this example, the hydrogen peroxide reactor described in Example 2 was used for the production test. In this embodiment, the operating condition of a fixed constant voltage of 10V was adopted. The current density of the reactor was calculated and the result was 2.08 mA / cm 2 .
[0084] Specifically:
[0085] After the air diffuses to the opening of the cathode end plate 1-1 of the reactor, it passes through the diffusion layer and the current collector substrate of the gas diffusion cathode 2 in sequence, and a reduction reaction occurs on the catalytic layer.
[0086] A 0.05-0.2 mol / L sulfuric acid aqueous solution is configured. In this embodiment, a 0.1 mol / L sulfuric acid aqueous solution is transported to the anode reaction chamber through the water inlet at the upper end of the anode end plate 1-2 to undergo an oxidation reaction on the surface of the Pt anode 6 .
[0087] Oxygen is reduced at the cathode to produce HO 2 - , the H produced by the anodic oxida...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com