Preparation method of migration-resistant fluorescent organic silicon elastomer
A silicone and elastomer technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of easy aggregation and crystallization of fluorescein, and achieve the effects of saving raw materials, reducing manufacturing costs, and increasing luminous intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Synthesis of polymerizable small molecule fluorescent units in this example:
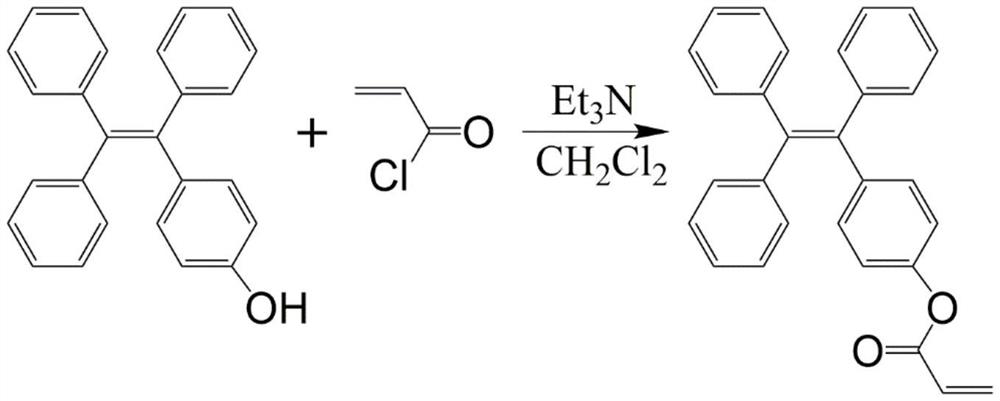
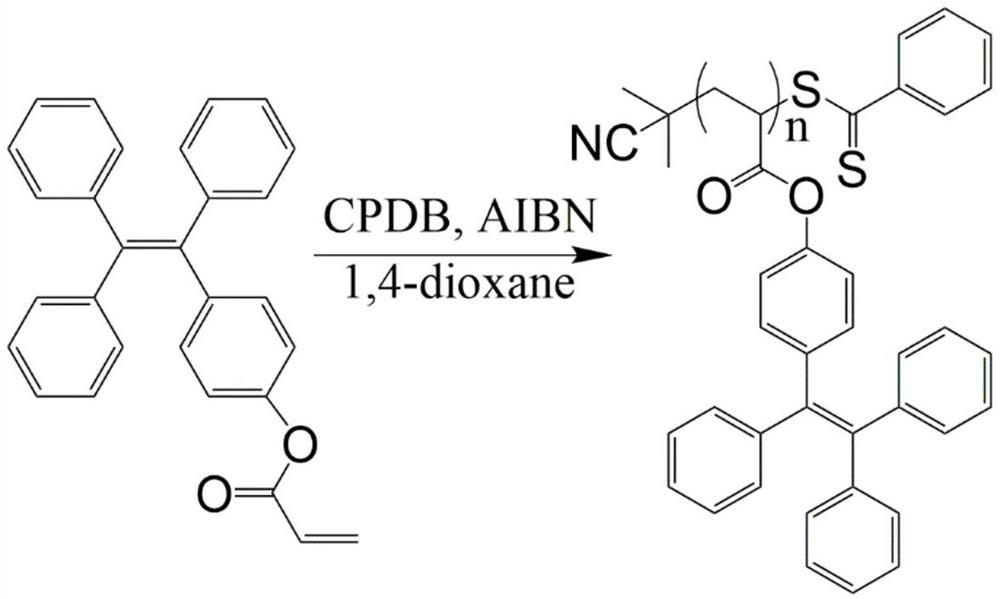
[0032] In a 100mL three-neck flask washed and dried, dissolve monohydroxytetraphenylethylene (TPE-OH, 1.0g, 2.87mmol) and triethylamine (0.544mL, 4.305mmol) in 40mL of dichloromethane, and place In a cold water bath, a solution of acryloyl chloride (0.354 mL, 4.305 mmol) in dichloromethane (20 mL) was slowly added dropwise under nitrogen atmosphere. The reaction mixture was stirred in a cold water bath for 3 min, and then stirred at reflux for 2 h at room temperature. The reaction solution was transferred to a 125mL separatory funnel, washed three times with 15mL water and twice with 20mL saturated brine, and the organic phase was concentrated to 2-3mL by rotary evaporation. Column chromatography (silica gel 200-300 mesh) separation, using a mixed solvent of dichloromethane and petroleum ether (8 / 1, V / V) as eluent, rotary evaporation to dryness, then placed in a 35 ° C vacuum oven to dry...
Embodiment 2
[0044] (1) Synthesis of macromolecular fluorescent compounds in the present embodiment:
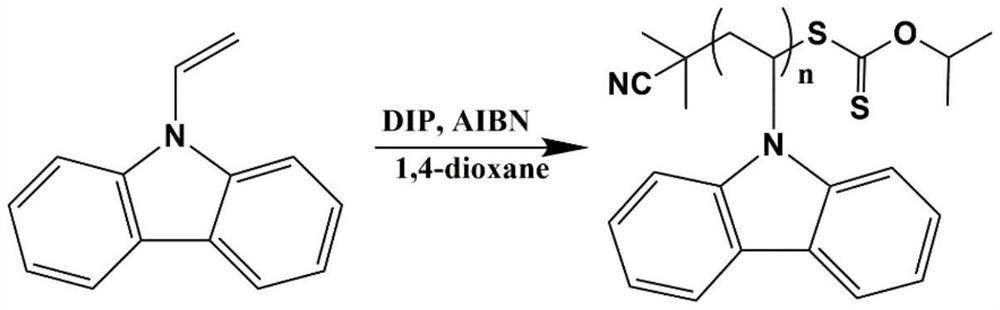
[0045] Weigh 1g (0.005mol) of vinyl carbazole, 24.6mg (0.15mmol) of initiator azobisisobutyronitrile into a 10mL Schlenk reaction tube, weigh 27mg (0.1mmol) of chain transfer agent diisopropyl yellow disulfide The orthoester was dissolved in 2 mL of 1,4-dioxane, injected into a Schlenk reaction tube, stirred and sonicated. Freezing with liquid nitrogen-vacuumizing-argon filling-thawing cycle was performed three times, and then stirred and reacted at 70° C. for 3 h under a nitrogen atmosphere. Stop the reaction, cool down to room temperature, add a small amount of 1,4-dioxane dissolved product, and stir evenly. The reaction solution was added dropwise into 60mL of cold methanol and stirred continuously to obtain a white precipitate, 10000r·min -1 Centrifuge for 10 minutes. Then it was dissolved in THF, precipitated in cold methanol, and purified by centrifugation once. The product was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com