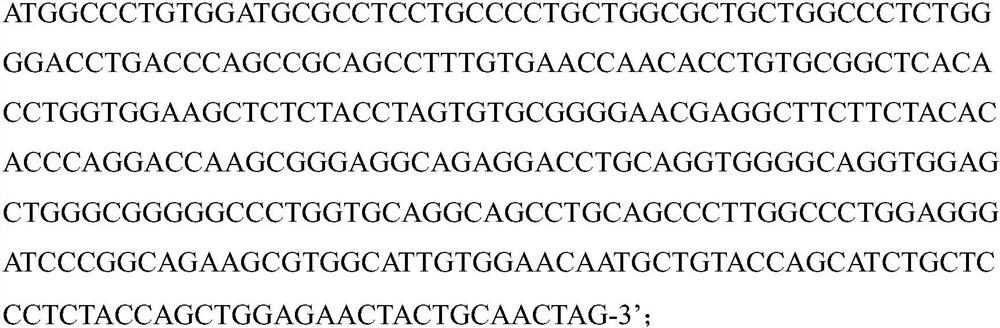IRES-hINS recombinant gene, lentivirus, and construction method and application of IRES-hINS recombinant gene
A technology of recombining genes and genes, applied in the direction of reverse transcription RNA virus, application, virus, etc., can solve the problems of low proportion of functional cells, poor activity, difficult to achieve insulin secretion efficiency, etc., achieving huge social and economic benefits, and small side effects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The construction of embodiment 1 lentiviral vector
[0038] According to the GenBank database (http: / / www.ncbi.nlm.nih.gov / genbank), the mRNA sequence, P2A sequence, Puro sequence and ires sequence of A chain and B chain of human insulin gene were obtained.
[0039] We further transformed the human insulin gene into gene sequences of insulin α and β chains containing A, B chains and furin cleavage sites. The sense chain of the oligonucleotide sequence of the hINS sequence is:
[0040]
[0041]
[0042] The antisense strand is:
[0043]
[0044] P2A:
[0045] The justice chain is:
[0046]
[0047] The antisense strand is:
[0048]
[0049] Ires (IRES FGF ):
[0050] The justice chain is:
[0051]
[0052]
[0053] The antisense strand is:
[0054]
[0055] The sequence described in the present invention was synthesized by Sangon Bioengineering (Shanghai) Co., Ltd. according to the sequence of Ires-P2A-Insulin. The above chain oligonucleotid...
Embodiment 2I
[0059] The preparation of embodiment 2IRES-hINS, hINS and hINSnf lentivirus
[0060] The lentivirus was packaged according to the instructions of Lipofectamine 2000 reagent. The cells transfected with the lentiviral vector prepared in Example 1 were used as the experimental group, while the cells transfected with the pLVX lentiviral empty vector were used as the control group.
[0061] Referring to the lipofectmin2000 reagent instructions, transfer the target plasmid and helper plasmids pMdlg, RSV-REV, and VSV-G into 293FT cells, prepare and collect the virus, and then infect 293FT with lentivirus containing IRES-hINS, bINS, and hINSnf sequences, and use Puromycin Puromycin was used to select resistant cells for experiments.
[0062] The specific steps are as follows: 0.75 μg pMdlg, 0.35 μg RSV-REV, 0.49 μg VSV-G, 0.61 μg lentiviral vectors Plvx-RES-hINS, pLVX-hINS and pLVX-hINSnf were added to 0.5 mL of low serum OPTI-MEM In the culture medium, mix gently and incubate at ro...
Embodiment 3I
[0063] Example 3 IRES-hINS, hINS and hINSnf lentiviral transfection of 293T cells
[0064] Use the IRES-hINS, hINS and hINSnf lentiviruses collected in Example 2 to transfect 293FT cells respectively: on the first day, plant the plate, count the cells, and adjust the cell density to one-third of the bottom area of the 6cm culture dish; on the second day , virus infection, take out the 6cm petri dish, discard the supernatant, mix the virus and 293FT cell culture medium at a volume ratio of 1:1, and add 8mg / mL polybrene (polybrene) to make the effect concentration 8ug / mL, put in Incubate for 8 hours in an incubator; after 8 hours of incubation, discard the supernatant, change the medium for the cells, and continue to culture in the incubator for two days; after culturing for two days, discard the supernatant and dilute with 1000 mg / mL puromycin at a volume ratio of 1:1000 , screening for 2-3 days; re-seeding: Digest the cells after two days of screening and plant them back int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com