Human anti-Siglec-15 antibody and application thereof
An antibody and sequence technology, applied in the field of human anti-Siglec-15 antibody, can solve the problems of unclear immune function and achieve the effect of increasing diversity, high binding activity and long half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Screening of Siglec-15 fully human phage antibody library
[0039] Dissolve 10 μg of Siglec-15 protein in 500 μL of PBS and add to the immunotube to coat overnight at 4°C, and set a control tube without antigen. Pour off the coated immunotube liquid and seal it with 4% milk at room temperature for 1h, and at the same time, put 5×1012 A natural phage library was diluted in 1 mL containing 4% milk and blocked for 1 h at room temperature. The blocking solution in the immunotube was discarded, and 500 μL of the blocked phage library solution was added to the antigen-coated immunotube and the control tube respectively, and incubated at room temperature for 1 h. Use PBST (0.1% Tween 20 added to PBS) to wash the immunotube 25 times to wash away unbound and non-specifically bound phages, and elute specifically bound phages with glycine-hydrochloric acid (100mM, pH1.7), Tris base After neutralization (pH 8.0), add to TG1 in the logarithmic growth phase and incubate at...
Embodiment 2E
[0040] Embodiment 2 ELISA method identifies positive clone binding activity
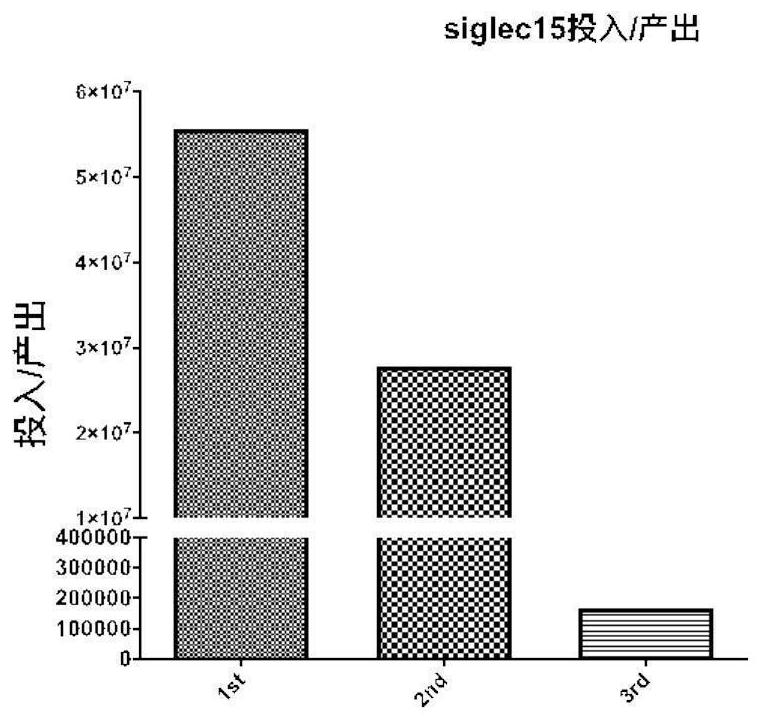
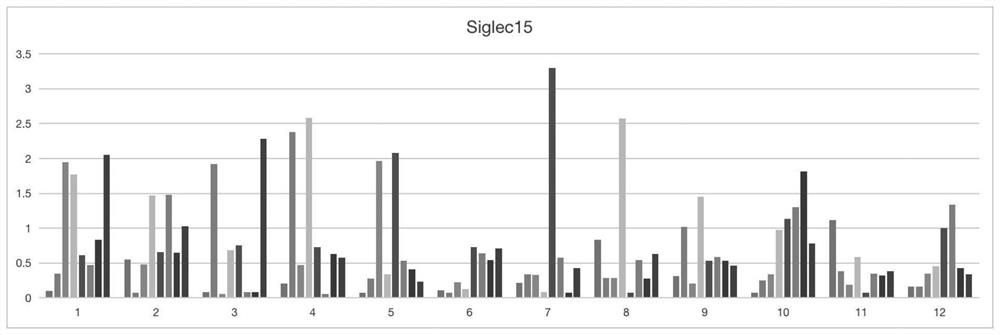
[0041] Dilute siglec-15 protein with coating solution (0.1M sodium carbonate-sodium bicarbonate buffer (pH9.6)) to 1 μg / mL, add 100 μL per well to the ELISA strip, overnight at 4°C; wash the plate with PBST washing solution 3 Add 200 μL 4% skimmed milk powder to each well, block for 1 hour at 37°C, wash the plate 3 times; centrifuge the phage supernatant collected above at 1800r / min 4°C for 15 minutes, add 100 μL to each well into the ELISA plate, and react for 1 hour at 37°C ; wash the plate 3 times; add 100 μL anti-M13 / HRP to each well, and react in the dark at room temperature for 45 minutes; wash the plate 4 times; add 100 μL TMB substrate chromogenic solution to each well, and react in the dark at room temperature for 3 minutes; 2 SO 4 The reaction was terminated, and the OD450 value was detected with a microplate reader; the results showed that most of the clones had good binding activity, and...
Embodiment 3
[0042] Example 3 Antibody expression and identification
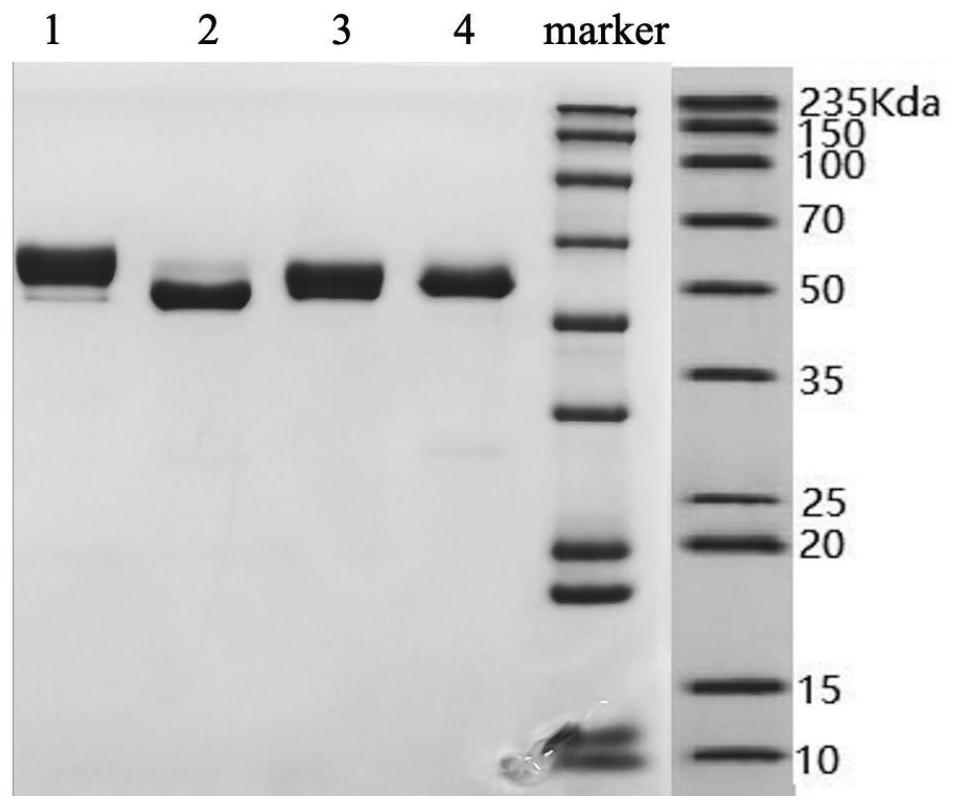
[0043] The antibody heavy chain and light chain variable regions and human Fc constant region gene sequences obtained by screening were cloned into the commercial vector pCDNA3.4, the correctness of the clones was identified by enzyme digestion, and the plasmids were extracted using a plasmid extraction kit. Resuscitate 293T cells in advance, when the cells are in the logarithmic growth phase, use JetPRIME transfection reagent to transfect the antibody expression vector into 293T cells, change the complete medium for 4 hours and continue to culture for 12 hours, change to OPM-293CD05 medium and continue to culture After 72 hours, the supernatant was collected by centrifugation, filtered with a 0.22 μM filter element, purified with a Protein A chromatography column, and identified by SDS-PAGE and SEC-HPLC. The antibody molecular weight was about 60Kda under SDS-PAGE reducing conditions, and the antibody purity was > 95%,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com