Preparation method of light-driven molecular motor based on oxidized benzofuran structure
A technology of light-driven molecules and molecular motors, which is applied in the preparation of carbon-based compounds, the preparation of organic compounds, and the preparation of aminohydroxy compounds, etc., can solve the problems of degradation, incompatible wavelengths, low pollution and convenience, and achieves less waste, less waste, The effect of low environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
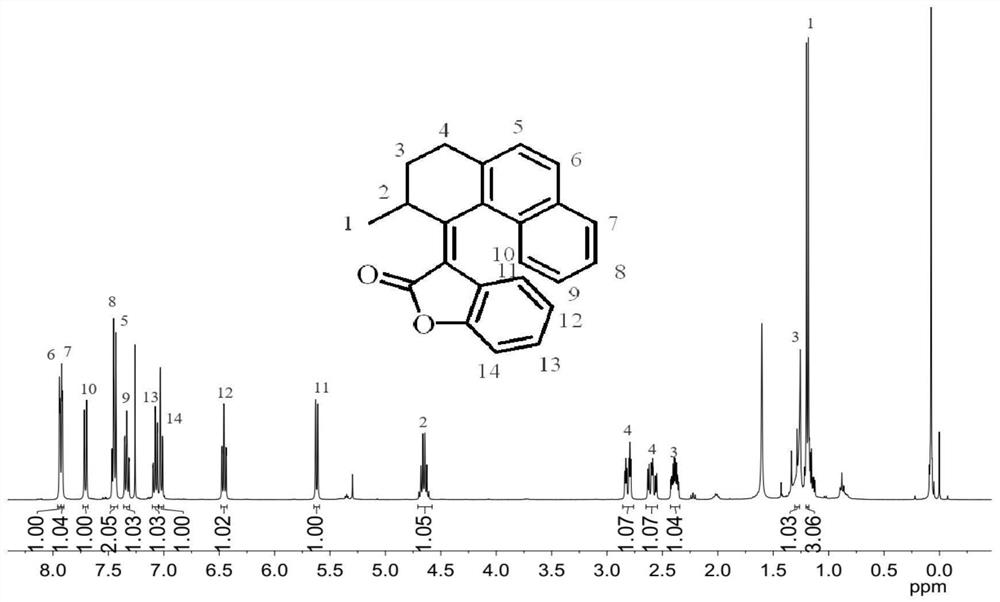
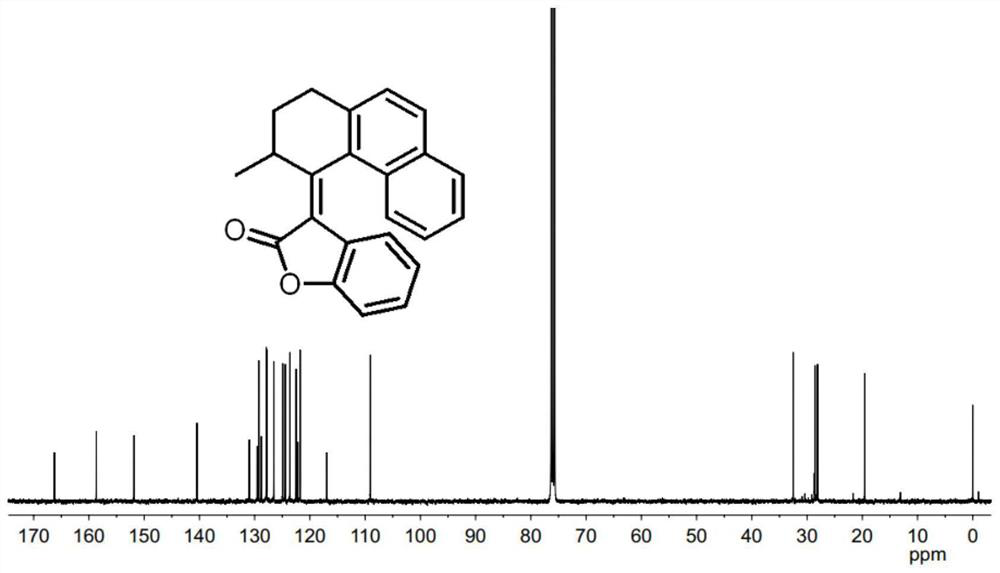
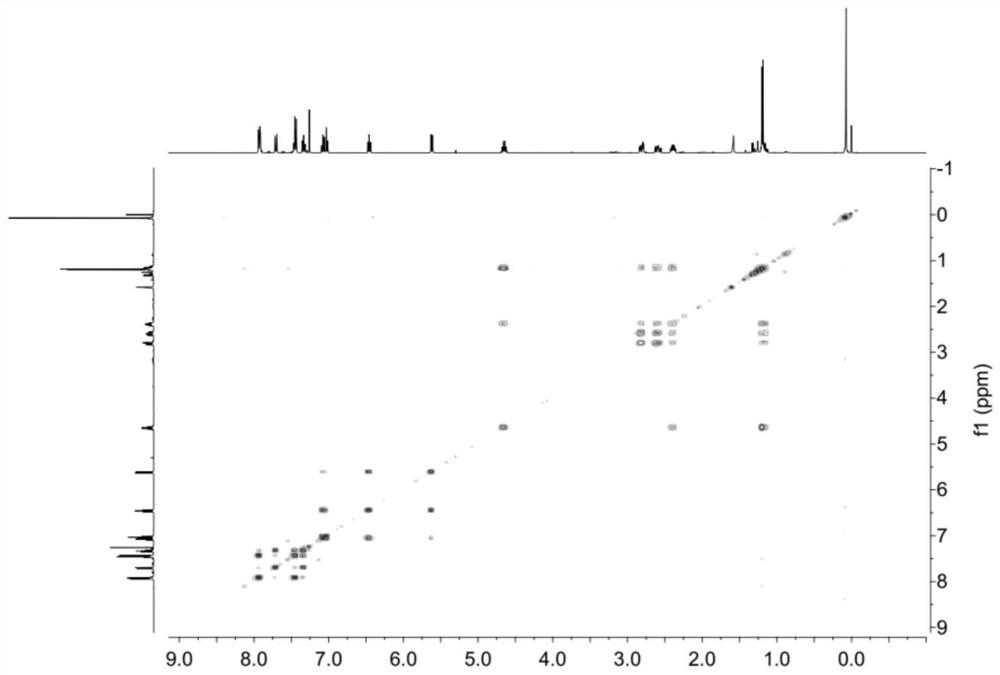
[0074] Synthesis of Compound 4: Add AlCl to a 100mL flask under nitrogen protection 3 (8g, 0.06mol, 2.0eq.) and nitrobenzene (PhNO 2 , 25mL), stirred until dissolved, then added succinic anhydride (3g, 0.03mol, 1.0eq.) and naphthalene (5.8g, 0.045mmol, 1.5eq.), stirred at room temperature overnight. After the TLC detection reaction was completed, ice water (20mL) and 6mol / L hydrochloric acid (5mL) were added, and a pale yellow precipitate was precipitated. The mixture was filtered, and the filter cake was rinsed 3 times with n-hexane (30mL) and water (30mL) (10mL ×3). Toluene (20 mL) was added to a 100 mL single-necked bottle containing the above filter cake, heated to 65° C. and stirred for 0.5 h, cooled to 35° C., filtered, and rinsed with a little toluene to obtain compound 4 (off-white solid, 2.54 g). The rate is 37%. 1 H NMR (400MHz, CDCl 3 )δ8.52(s,1H),8.05(d,J=8.4Hz,1H),7.97(d,J=8.0Hz,1H),7.89(t,J=8.6Hz,2H),7.61(t, J=7.2Hz, 1H), 7.56(t, J=7.4Hz, 1H), 3.47(t, J=6.6H...
Embodiment 2
[0079] Synthesis of Molecular Motor 1: Under the protection of nitrogen, add compound 2 (100mg, 0.47mmol, 1.0eq) and anhydrous THF (3mL) into a 25mL two-necked flask, cool to 0°C, add titanium tetrachloride (TiCl 4 , 0.1mL, 0.7mmol, 1.5eq), the dropwise addition was completed and stirred for 5min, then added 2-coumaranone 3 (95mg, 0.7mmol, 1.5eq) dissolved in THF (2mL) and 1,8-di Azabicycloundec-7-ene (DBU, 0.15 mL, 0.7 mmol, 1.5 eq). Wait until it rises to room temperature and react for 5h. Add 10 mL of hydrochloric acid (1mol / L) to the reaction mixture to quench, extract with ethyl acetate (50 mL×3), combine the organic phases, wash with saturated brine and dry over anhydrous sodium sulfate, and concentrate the organic phases under reduced pressure to obtain a reddish-brown oily substance, crude Motor 1 (yellow solid, 118 mg) was separated by thin layer chromatography with a yield of 76%. 1 H NMR (400MHz, CDCl 3 )δ7.94-7.92(m,2H),7.71(d,J=8.4Hz,1H),7.47-7.44(m,2H),7.36-7....
Embodiment 3
[0081] The light-driven process of molecular motor 1: when (P)-(S)-stable E-1 is irradiated with a 365nm light source, it will cause photoisomerization of the double bond in the middle of the axis, and generate (M)-(S)-unstable Z-1, which can undergo irreversible thermally induced helical inversion (THI) at room temperature (25°C) to form (P)-(S)-stable Z-1, completes the first 180° rotation. Similarly, (P)-(S)-stable Z-1 was irradiated with a 365 nm light source to form (M)-(S)-unstable E-1, which undergoes irreversible thermally induced helical inversion (THI) at 80 °C and then Obtain initial (P)-(S)-stable E-1. After the second rotation cycle is completed, the upper half of the motor completes a 360° unidirectional rotation relative to the lower half.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com