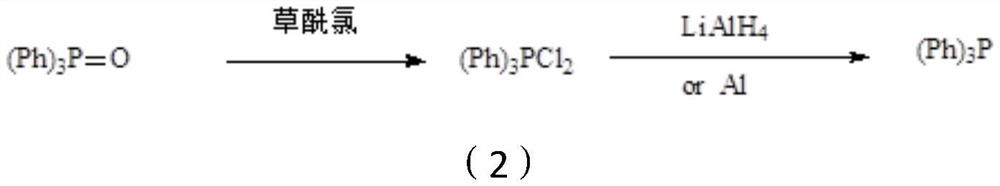
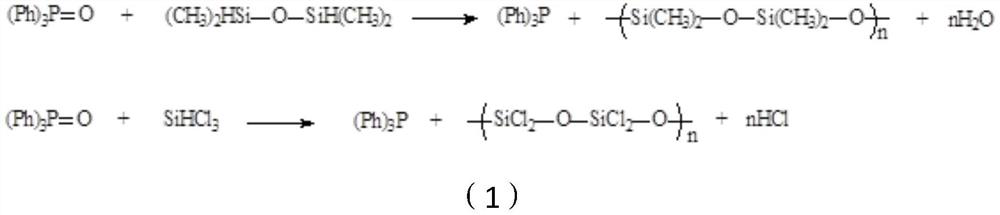Preparation method of triphenylphosphine
A technology of triphenylphosphine and triphenylphosphine dichloride, applied in the field of triphenylphosphine preparation, can solve the problems of high process risk, high market price, excess active aluminum powder and the like, and achieves high process safety, The effect of sufficient market supply and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
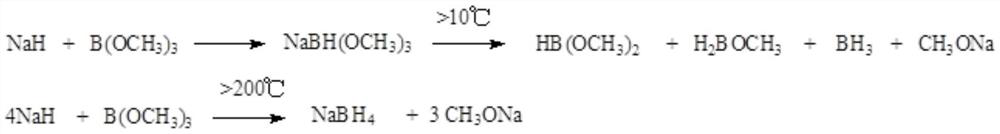
[0059] The embodiment of the present invention provides the present invention provides a kind of preparation method of triphenylphosphine, comprises the following steps:
[0060] Sodium trimethoxy borohydride is prepared by reacting trimethoxy borohydride sodium with triphenylphosphine dichloride to obtain triphenylphosphine.
[0061] It also includes: reacting sodium hydride and trimethyl borate to obtain a reaction solution of sodium trimethoxy borohydride; adding the obtained reaction solution of sodium trimethoxy borohydride dropwise to solution 1 to react to obtain triphenyl base phosphine. Described solution one is the triphenylphosphine dichloride solution containing catalytic amount of aluminum trichloride or stannous chloride.
[0062] It also includes: adding sodium hydride and solvent A to the reaction bottle 1; then cooling down to -50-10°C under the protection of nitrogen, and stirring evenly at -50-10°C under the condition of heat preservation, and then adding s...
experiment example 1
[0105] 1.1. Add 20 g (0.5 mol) of 60% sodium hydride and 80 g of anhydrous tetrahydrofuran into a 250 ml reaction bottle one. Under nitrogen protection, stir and cool down to 0-10°C. Then maintain nitrogen protection, stirring and liquid temperature 0-10°C, dropwise add 57.2g (0.55mol) of trimethyl borate; after the dropwise addition, continue to keep warm at 0-10°C and stir for 1 hour.
[0106] 1.2. Add 74.9g (0.225mol) of triphenylphosphine dichloride and 300g of tetrahydrofuran to another 1000ml reaction flask II, and control the temperature at 0-10°C under nitrogen protection and stirring. Then first add 0.375g of stannous chloride; then stir at 0-10°C under temperature control, and dropwise add the reaction mixture obtained in 4.1.1. After the dropwise addition, continue to keep warm at 0-10°C for 15 hours. Then, it was filtered at room temperature to remove the insoluble matter (sodium chloride) produced by the reaction.
[0107] 1.3, the filtrate gained in 1.2 is con...
experiment example 2
[0109] 2.1. Add 20 g (0.5 mol) of 60% sodium hydride and 120 g of anhydrous 2-methyltetrahydrofuran into 1000 ml reaction flask one. Under the protection of nitrogen, stir and cool down to -50~-40°C. Then maintain nitrogen protection, stirring and liquid temperature -50~-40°C, dropwise add a mixture of 208g (2mol) of trimethyl borate and 200g of 2-methyltetrahydrofuran; continue to keep warm at -50~-40°C after the dropwise addition Stir for 5 hours.
[0110] 2.2. Add 66.6g (0.2mol) of triphenylphosphine dichloride and 533g of 2-methyltetrahydrofuran / trimethyl borate mixture to another 2000ml reaction flask II, and control under nitrogen protection and stirring. Temperature -20~-10℃. Then add 3.33g of anhydrous aluminum trichloride first; then stir at -20 to -10°C under temperature control, and dropwise add the reaction mixture obtained in 4.2.1. After the dropwise addition, continue to keep warm at -20~-10°C for 3 hours. Then filter at -20 to -10°C to remove the insoluble ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com