4'-N-demethyl-vicenistatin as well as preparation method and application thereof
A methyltransferase and drug technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, applications, etc., can solve the problems of increasing the probability of repeated discovery of strains and secondary metabolites, and difficulty in research and development of microbial-derived drugs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
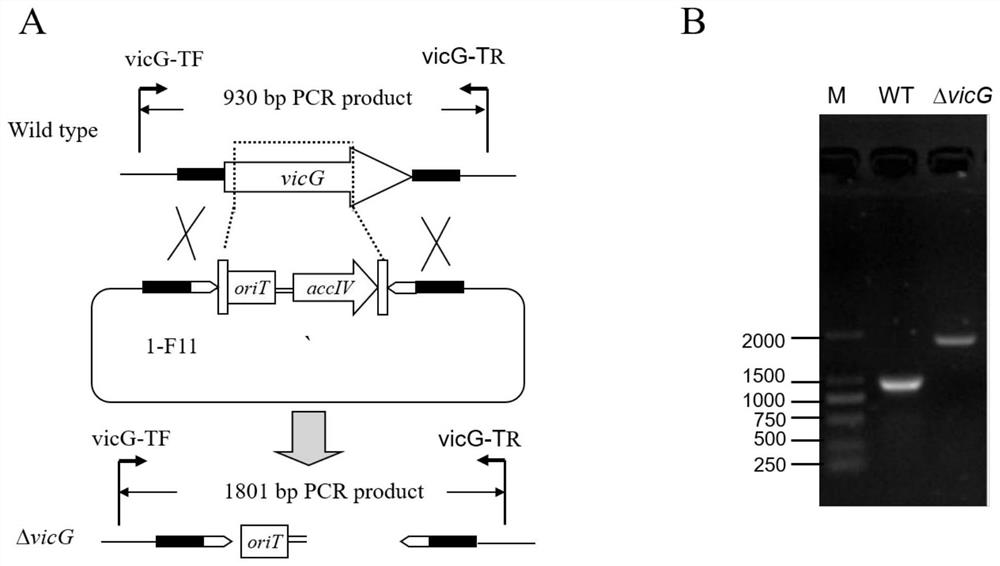
[0044] Construction of embodiment 1 methyltransferase gene mutant strain SCSIO Mla-L010 / ΔvicG
[0045] 1. Construction of S.parvus SCSIO Mla-L010 Genomic Library and Screening of Cosmids Containing Methyltransferase Genes
[0046] 1.1 Construction of S.parvus SCSIO Mla-L010 Genomic Library
[0047] The S.parvus SCSIO Mla-L010 genomic library was constructed with reference to the SuperCos1 Cosmid Vector Kit (Agilent) and Gigapck III XL packingExtract (Epicentre) operating manuals. After submerged culture of S.parvus SCSIO Mla-L010 strain for 2 days, the cells were collected by centrifugation, and genomic DNA was extracted by phenol-chloroform extraction. Take a suitable concentration of high-purity DNA and add a certain concentration of Sau3AI enzyme, and react for a certain period of time to digest the genomic DNA into a suitable fragment size, which can be tested on a nucleic acid gel, and the digested product is extracted and purified with phenol-chloroform and dephosphoryl...
Embodiment 2
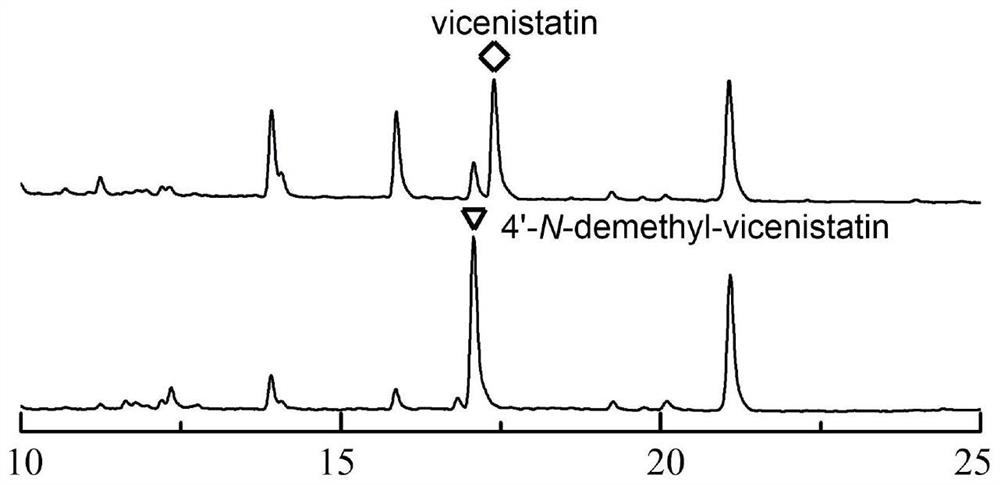
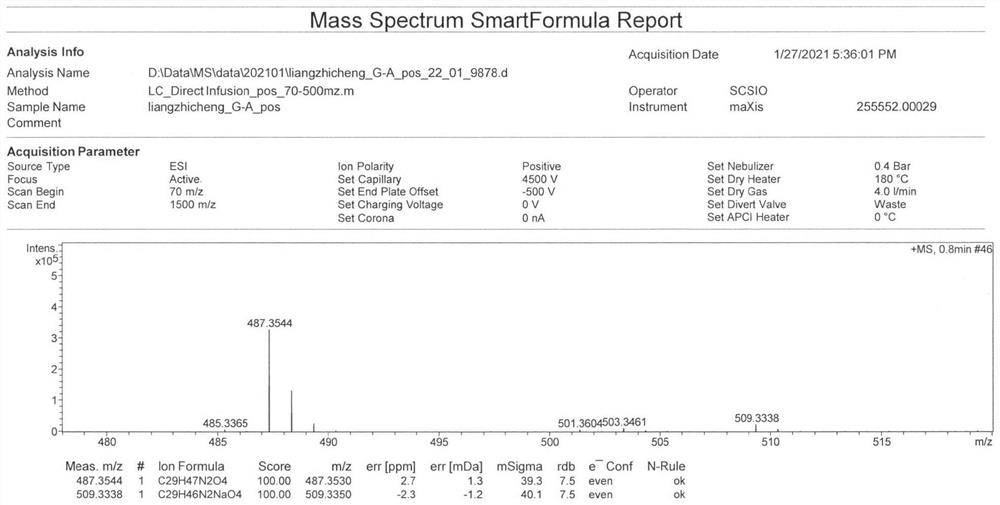
[0062] Example 2 Separation and structure identification of 4'-N-demethyl-vicenistatin
[0063] The fermentation culture of the 4'-N-demethyl-vicenistatin production bacterial strain SCSIO Mla-L010 / ΔvicG was prepared using the scale fermentation conditions described in Example 1, and the mycelium precipitate and fermentation surface were separated by centrifugation (4000r / min, 15min). Serum. Add 3 times the volume of acetone to the mycelium precipitate for extraction, repeat the extraction 3 times, and concentrate the extract under reduced pressure to form an extract. An equal volume of butanone was added to the fermentation supernatant for extraction three times, and the extract was concentrated under reduced pressure to form a concentrated extract. Combine the above two parts of concentrated extracts, add silica gel to mix the sample, and then perform normal phase silica gel column chromatography, and use chloroform / methanol system to carry out gradient elution according to...
Embodiment 3
[0067] Example 3 4'-N-demethyl-vicenistatin measures the minimum inhibitory concentration of a series of active indicator strains
[0068] The antibacterial activity of vicenisatin and 4'-N-demethyl-vicenistatin was evaluated by the broth microdilution method. Dissolve vicenisatin, 4'-N-demethyl-vicenistatin, and positive controls vancomycin (Vancomycin), ampicillin (Ampicillin), and amphotericin B (Amphotericin B) in dimethyl sulfoxide (DMSO) to prepare the final The drug solution with a concentration of 1.6mg / mL should be stored in a -20°C refrigerator for later use.
[0069] Select Mueller-Hinton (MH) broth liquid medium to cultivate the active test strain, its formula is as follows beef extract powder 300g / L, soluble starch 15g / L, casein acid hydrolyzate 17.5g / L, adjust the pH value to 7.3, Sterilize at 121°C for 30 minutes for later use.
[0070]Dilute the overnight cultured test bacteria solution with MH medium until the absorbance value at 600nm wavelength is approxim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com