Catalytic oxidation catalyst for removing chlorine-containing VOCs and preparation method of catalytic oxidation catalyst
A catalytic oxidation and catalyst technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, catalyst carrier, etc., can solve the problem of poor thermal stability at high temperature, anti-chlorine poisoning ability and anti-carbon poisoning Insufficient capacity and high activation temperature, achieving the effects of strong carbon poisoning ability, excellent low temperature activity, and strong resistance to Cl poisoning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
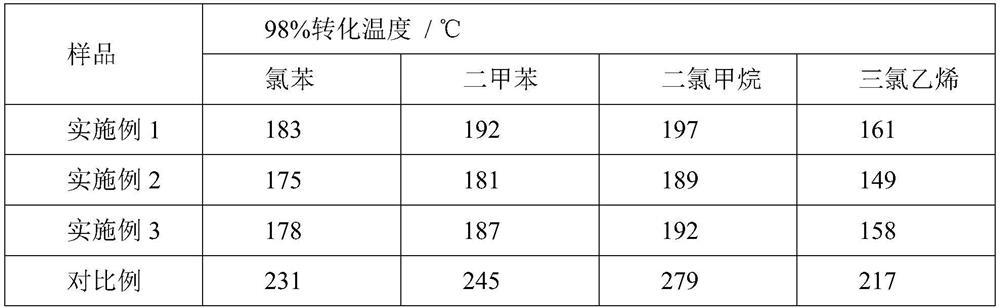
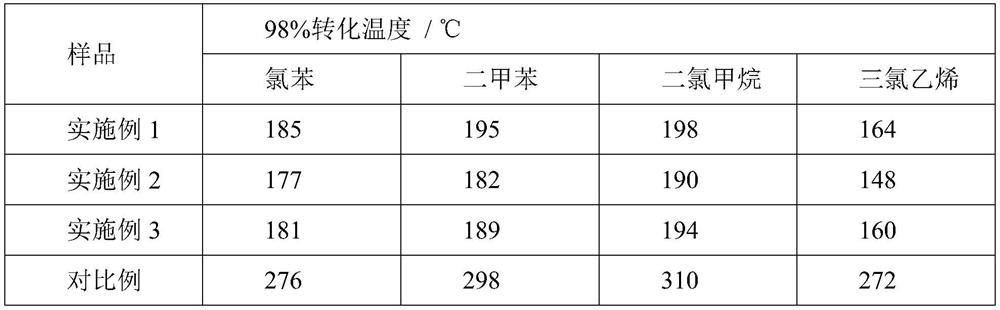
Examples
Embodiment 1
[0025] The preparation process of the present embodiment catalytic oxidation catalyst is as follows:
[0026] (1) Preparation of hydroxyapatite carrier
[0027] Dissolve 1312g Ca(NO 3 ) 2 and 712.8g (NH 4 ) 2 HPO 4 , add ammonia water to both solutions to adjust the pH value to 10; under vigorous stirring conditions, (NH 4 ) 2 HPO 4 5% of the solution was added to the Ca(NO 3 ) 2 In the solution, after a small amount of precipitation appears in the solution, the remaining (NH 4 ) 2 HPO 4 All the solution was added, and after the reaction was completed, it was left standing at 40°C for 24 hours; the precipitate was obtained by filtration, washed with water and ethanol, and dried to obtain 799g Ca 10 (PO 4 ) 6 (OH) 2 Hydroxyapatite carrier.
[0028] (2) Ce-Zr-La-PrO x Preparation of Composite Metal Oxide
[0029] Prepare 10L of citric acid aqueous solution of 0.5mol / L, add 67.14g Ce(NO 3 ) 3 ·6H 2 O, 116gZrOCl 2 ·8H 2 O, 155.9g La(NO 3 ) 3 ·6H 2 O and 3...
Embodiment 2
[0035] The preparation process of the present embodiment catalytic oxidation catalyst is as follows:
[0036] (1) Preparation of hydroxyapatite carrier
[0037] Dissolve 1572.1g Mg(NO 3 ) 2 and 839.5g (NH 4 ) 2 HPO 4 , add ammonia water to both solutions to adjust the pH value to 10; under vigorous stirring conditions, (NH 4 ) 2 HPO 4 7% of the solution was added to Mg(NO 3 ) 2 In the solution, after a small amount of precipitation appears in the solution, the remaining (NH 4 ) 2 HPO 4 Add all the solution, and let it stand at 25°C for 12 hours after the reaction; filter to obtain a precipitate, wash with water and ethanol, and dry to obtain 890g of Mg 10 (PO 4 ) 6 (OH) 2 Hydroxyapatite carrier.
[0038] (2) Ce-Zr-La-PrO x Preparation of Composite Metal Oxide
[0039] Prepare 7L of citric acid aqueous solution of 2mol / L, add 37.3g Ce(NO 3 ) 3 ·6H 2 O, 64.45g ZrOCl 2 ·8H 2 O, 86.6g La(NO 3 ) 3 ·6H 2 O and 8.7g Pr(NO 3 ) 3 ·6H 2 O, then add concentra...
Embodiment 3
[0044] The preparation process of the present embodiment catalytic oxidation catalyst is as follows:
[0045] (1) Preparation of hydroxyapatite carrier
[0046] Dissolve 1397.3g Ca(NO 3 ) 2 and 656g (NH 4 ) 2 HPO 4 , add ammonia water to both solutions to adjust the pH value to 10; under vigorous stirring conditions, (NH 4 ) 2 HPO 4 4% of the solution was added to the Ca(NO 3 ) 2 In the solution, after a small amount of precipitation appears in the solution, the remaining (NH 4 ) 2 HPO 4 All the solution was added, and after the reaction was completed, it was left standing at 35°C for 20 hours; the precipitate was obtained by filtration, washed with water and ethanol, and dried to obtain 845g Ca 12 (PO 4 ) 7 (OH) 3 Hydroxyapatite carrier.
[0047] (2) Ce-Zr-La-PrO x Preparation of Composite Metal Oxide
[0048] Prepare 8L of citric acid aqueous solution of 1.6mol / L, add 57.1g Ce(NO 3 ) 3 ·6H 2 O, 98.6gZrOCl 2 ·8H 2 O, 132.5g La(NO 3 ) 3 ·6H 2 O and 16...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com