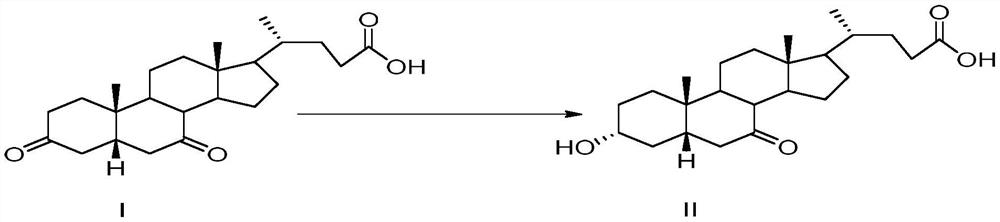
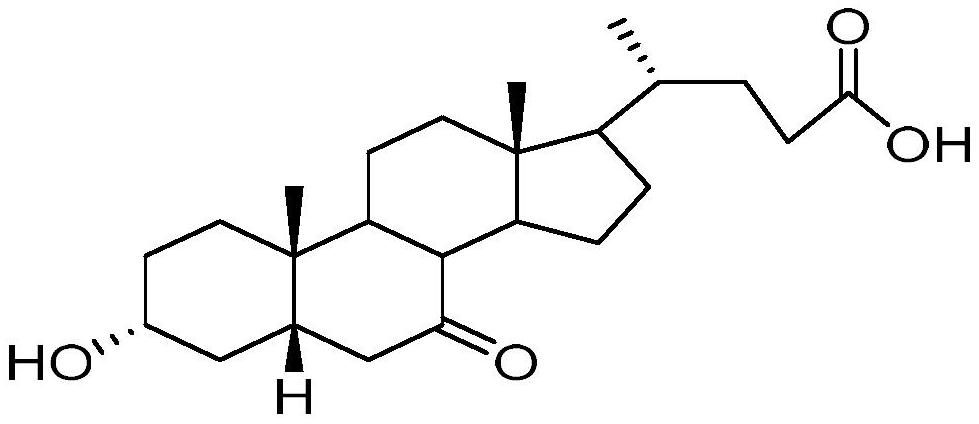
Preparation method of plant-derived 7-ketolithocholic acid
A cornerstone cholic acid and cornerstone technology, which is applied in the field of preparation of plant-sourced 7-ketolithocholic acid, can solve the problems of chenodeoxycholic acid and cholic acid source limitations, viruses brought into downstream products, and human impact, etc., to achieve reduction The effect of good effect, good purity and wide source
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 300ml of water in a clean reaction bottle, add 50.0g of compound I (purity: 99.1%) under stirring, adjust pH=13~14 with 2N sodium hydroxide, after compound I is completely dissolved, adjust pH= with 3N hydrochloric acid 8~9. Adjust the reaction temperature to 30-35° C., add 120 g of glucose, 10 g of 3α reductase, 22 g of glucose dehydrogenase, 1.5 g of coenzyme 1, and 1.5 g of coenzyme 2. After stirring evenly, use 2N sodium hydroxide solution to adjust the pH to 7-8 and react for 2 hours until the content of Compound I is 0.1% as monitored by HPLC. After the reaction is finished, the temperature of the system is raised to 70-80°C, and the temperature is kept and stirred for 30-60 minutes. Suction filtration while it is hot, collect the filtrate, heat the filter cake again to 70-80°C with 100ml of water, keep stirring for 30min-60min, heat filter and combine the filtrate. Cool the filtrate to 0-10°C, slowly add 2N hydrochloric acid dropwise to adjust pH = 2-3, a l...
Embodiment 2
[0027] Add 300ml of water to a clean reaction bottle, add 50.0g of compound I (purity: 99.1%) and 50.0ml of glycerin under stirring, adjust the pH to 7~8 with 2N sodium hydroxide, and adjust the reaction after compound I is completely dissolved At a temperature of 30-35°C, add 120g of glucose, 10g of 3α reductase, 22g of glucose dehydrogenase, 1.5g of coenzyme 1, and 1.5g of coenzyme 2. After stirring evenly, use 2N sodium hydroxide solution to adjust the pH to 7-8 and react for 2 hours until the content of Compound I is 0.1% as monitored by HPLC. After the reaction is finished, the temperature of the system is raised to 70-80°C, and the temperature is kept and stirred for 30-60 minutes. Suction filtration while it is hot, collect the filtrate, heat the filter cake again to 70-80°C with 100ml of water, keep stirring for 30min-60min, heat filter and combine the filtrate. Concentrate the filtrate under reduced pressure at 50-60°C to remove all glycerin, cool down to 0-10°C, slo...
Embodiment 3
[0029] Add 300ml of water into a clean reaction bottle, add 50.0g of compound I (purity: 99.1%) and 50.0ml of 2-methyltetrahydrofuran under stirring, adjust the pH to 7~8 with 2N sodium hydroxide, and wait until compound I is completely dissolved , adjust the reaction temperature to 30-35° C., add 120 g of glucose, 10 g of 3α reductase, 22 g of glucose dehydrogenase, 1.5 g of coenzyme I, and 1.5 g of coenzyme II. After stirring evenly, use 2N sodium hydroxide solution to adjust the pH to 7-8 and react for 2 hours until the content of Compound I is 0.1% as monitored by HPLC. After the reaction is finished, the temperature of the system is raised to 70-80°C, and the temperature is kept and stirred for 30-60 minutes. Suction filtration while it is hot, collect the filtrate, heat the filter cake again to 70-80°C with 100ml of water, keep stirring for 30min-60min, heat filter and combine the filtrate. Concentrate the filtrate under reduced pressure at 50-60°C to remove 2-methyltet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com