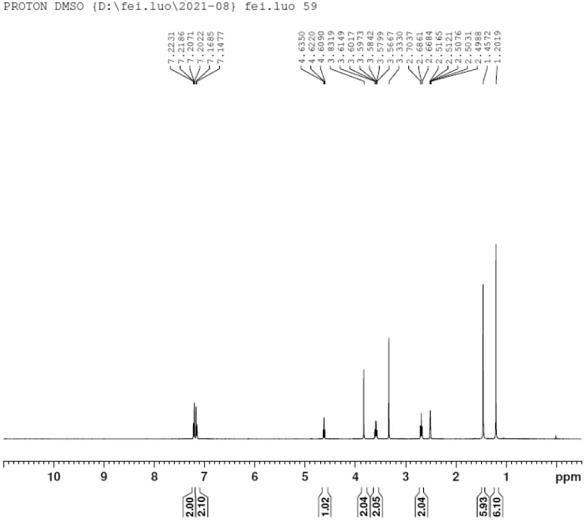
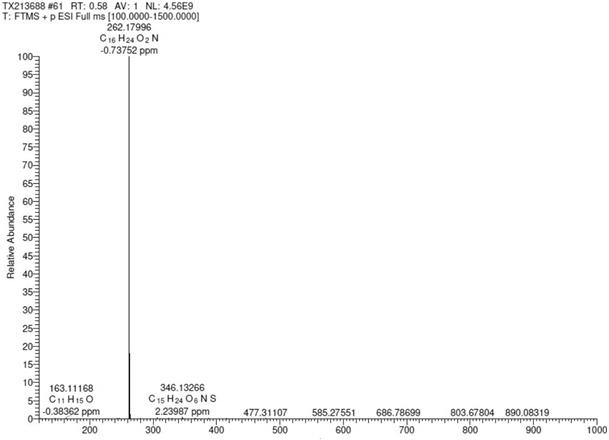
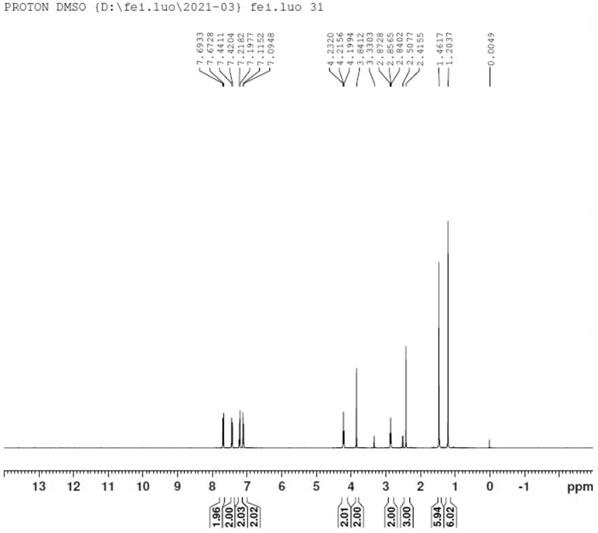
Preparation method of bilastine intermediate
A bilastine and intermediate technology, which is applied in the field of preparation of bilastine intermediates, can solve the problems of many side reactions, harsh reaction conditions, difficult operation and the like, and achieves reduced industrialization cost, mild reaction conditions, and avoidance of waste. The effect of acid waste gas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation of methyl 2-(4-(2-chloroacetyl)phenyl)-2-methylpropanoate
[0048] 126.7 g (1122 mmol) of chloroacetyl chloride, 300 mL of dichloromethane and 89.8 g (673 mmol) of anhydrous aluminum trichloride were successively added to the reaction flask, and the mixture was cooled to 0°C and stirred for 20 min, then dropped at 10°C Add 100 g (561 mmol) of the raw material 2,2-dimethylphenylacetic acid methyl ester, after the drop, raise the temperature to 30°C and stir for 2 hours. After the reaction is complete as monitored by TLC, add 300 mL of dichloromethane to dilute, and cool the reaction solution to 10°C , add 600 mL of water dropwise and stir to quench, then let stand for liquid separation, then add 600 mL of saturated NaHCO dropwise to the organic phase 3 The solution was washed once, and the organic phase was dried over anhydrous sodium sulfate. After filtration, the organic phase was concentrated to obtain 138.6 g of yellow oily matter, yield 97%, which was m...
Embodiment 2
[0050] Preparation of ethyl 2-(4-(2-bromoacetyl)phenyl)-2-methylpropanoate
[0051] Add 78.7 g (390 mmol) of bromoacetyl bromide, 150 mL of dichloromethane and 38.1 g (286 mmol) of anhydrous aluminum trichloride to the reaction flask in sequence, and the mixture is cooled to 0°C and stirred for 20 min, then dropwise at 0°C Add 50 g (260 mmol) of ethyl 2,2-dimethylphenylacetate as the raw material, and stir the reaction at 10°C for 1 h after dropping. After the reaction is complete as monitored by TLC, add 150 mL of dichloromethane to dilute, and cool the reaction solution to 0°C. Add 300 mL of water dropwise and stir to quench, then let stand to separate the liquid, then add 300 mL of saturated NaHCO dropwise to the organic phase 3 The solution was washed once, and the organic phase was dried over anhydrous sodium sulfate. After filtration, the organic phase was concentrated to obtain 74.1 g of a yellow oily substance, with a yield of 91%, which was ethyl 2-(4-(2-bromoacetyl)...
Embodiment 3
[0053] Preparation of methyl 2-(4-(2-chloroethyl)phenyl)-2-methylpropionate
[0054] 101.9 g (400 mmol) 2-(4-(2-chloroacetyl) phenyl)-2-methylpropionic acid methyl ester and 273.7 g (2400 mmol) trifluoroacetic acid were added to the three-necked flask and stirred, then 139.5 g (1200 mmol) triethylsilane was added, the mixture was heated to 50°C and stirred for 7 hours. After the reaction was monitored by TLC, it was cooled to 0°C, 800 mL of water was added to the reaction liquid to quench it, and extracted twice with dichloromethane. 400 mL each time, the organic phase was combined and washed once with 600 mL of water, and 600 mL of saturated NaHCO3 solution was washed once, and the organic phase was concentrated to dryness to obtain 96.3 g of yellow oil, with a yield of 94%, which was 2-(4-(2- Chloroethyl)phenyl)-2-methylpropanoic acid methyl ester;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com