Avibactam sodium substance analysis method
A technology of avibactam sodium and carbonyl avibactam sodium, which is applied in the direction of analyzing materials, material separation, measuring devices, etc., can solve the problems of short retention time, main peaks become double peaks, etc., and achieve good reproducibility, High stability and high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
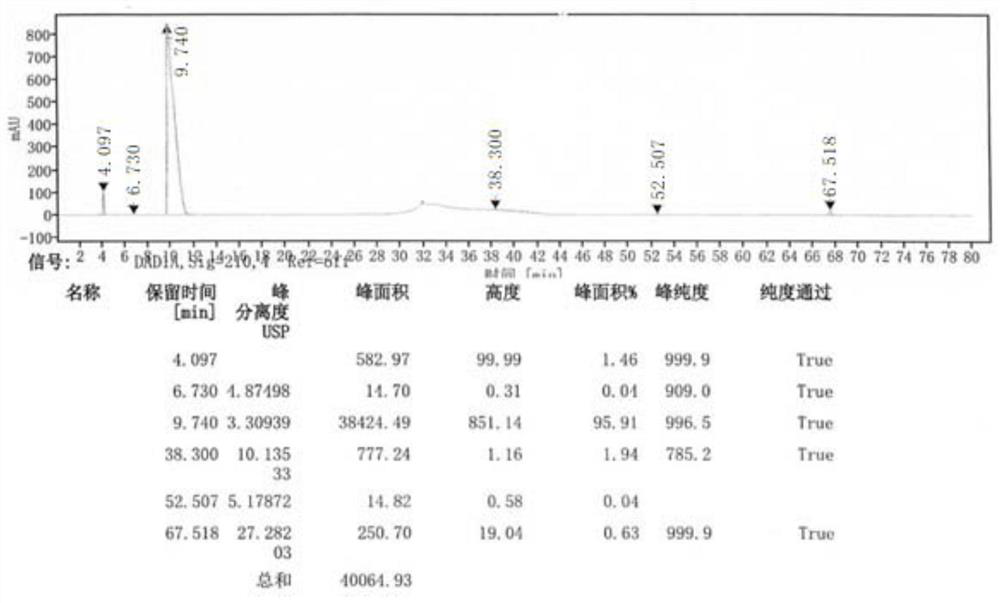
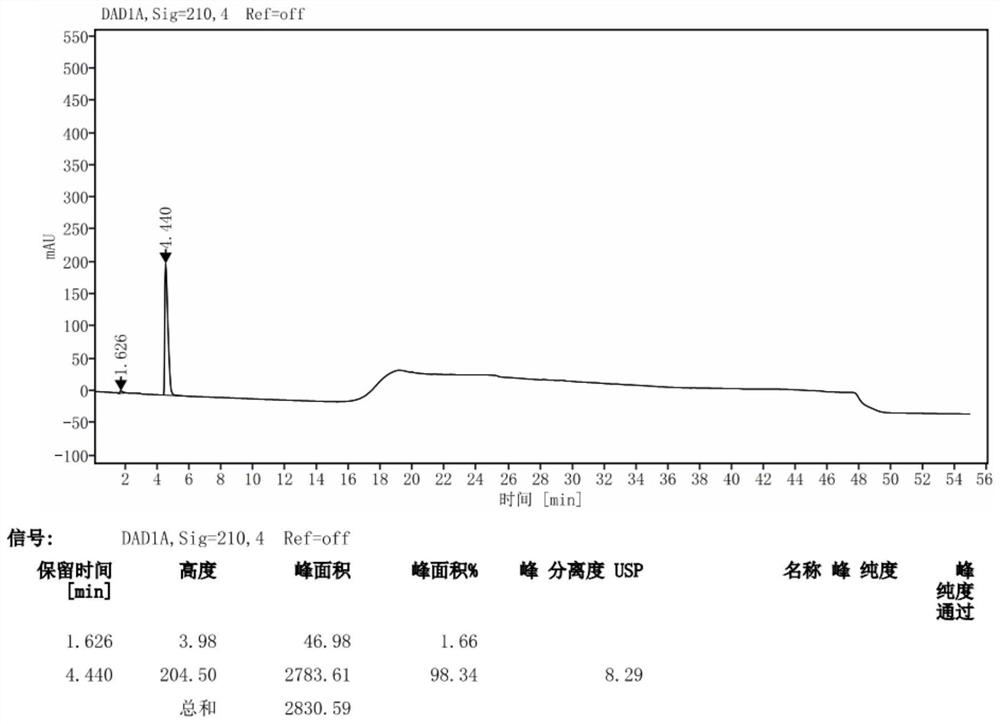
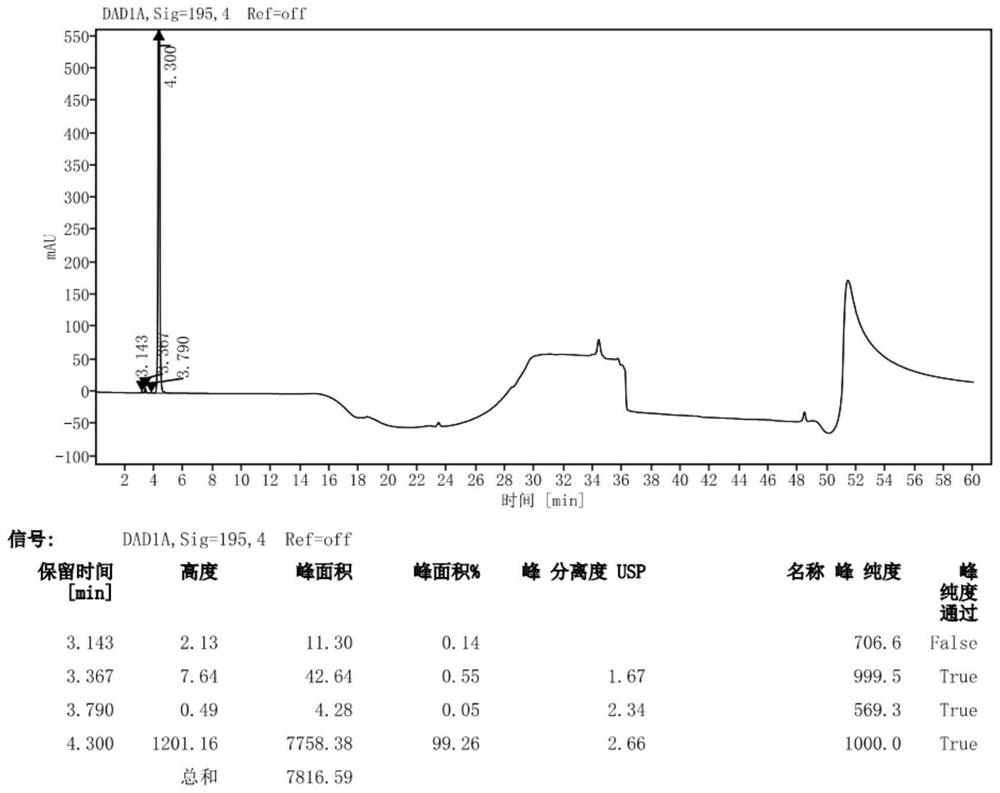
[0041] 1) Preparation of system suitability solution
[0042] Take 50mg of avibactam sodium, put it in a 25mL measuring bottle, add 1mL of 0.01mol / L sodium hydroxide solution, let it stand for 3 minutes, add 1mL of 0.01mol / L hydrochloric acid solution for neutralization, and then dilute to the mark with mobile phase A .
[0043] 2) Preparation of the test solution
[0044] Take 50mg of avibactam sodium, put it in a 25mL measuring bottle, add mobile phase A to dissolve and quantitatively dilute to the mark.
[0045] 3) Preparation of control solution
[0046] Take the above test solution, 1mL, put it in a 100mL measuring bottle, quantitatively dilute to the mark with mobile phase A, and shake well.
[0047] 4) Sensitivity solution preparation
[0048]Take 5mL of the above control solution, put it in a 100mL measuring bottle, quantitatively dilute to the mark with mobile phase A, and shake well.
Embodiment 2
[0050] This embodiment is the optimization and determination of the chromatographic conditions adopted by the analytical method of avibactam sodium
[0051] (1) Determination of detection wavelength
[0052] When the detection wavelength is 200nm, the response value is higher than that at 210nm, but because the organic solvent has terminal absorption, we choose to increase the sample concentration and detect at a wavelength of 210nm, and the signal-to-noise ratio of the sensitivity solution at this concentration is greater than 10, which meets the requirements .
[0053] (2) Optimal chromatographic conditions
[0054] Chromatographic column: Agilent SB-Aq C18 (250mm×4.6mm, 5μm), with ghost peak trapping Column: Mr. Chromatography (50mm); mobile phase A is 40mmol / L potassium dihydrogen phosphate solution, mobile phase B is 40mmol / L Potassium dihydrogen phosphate-acetonitrile (60:40), gradient elution; column temperature 35°C; flow rate 1.0mL / min; injection volume 20μl; detect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com