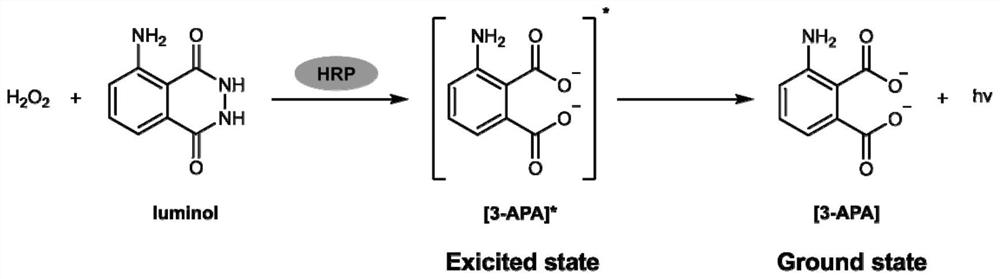
Application of ascorbic acid peroxidase 1 in catalysis of luminol chemiluminescence reaction
A peroxidase, ascorbic acid technology, applied in the direction of analysis, chemiluminescence/bioluminescence, etc. by chemical reaction of materials, which can solve problems such as limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Obtaining cAPX1 protein using prokaryotic expression system
[0044] 1. Reagent materials and equipment
[0045] Vector: pMAL-c2x-GW (gateway) - used for expression of MBP tagged protein;
[0046] Escherichia coli: BL21(DE3);
[0047] Column material: Amylose Resin, NEB, Cat # E8021L, for purification of MBP-tagged proteins;
[0048] IPTG: Solarbio, Cat # 18070, configured as a 240mg / mL (1M) aqueous solution, filtered and sterilized before storage, the use concentration is generally 0.2-0.5mM;
[0049] PBS buffer: store the stock solution at 20×, pH 7.2-7.6, Sangon Biotech, dilute to 1× with water when used;
[0050] Protease inhibitors: Merck, Cat # 4693116001, now add protein extract;
[0051] Empty chromatography column: 20mL, Sangon Biotech;
[0052] Protein electrophoresis apparatus, protein tank transfer apparatus, high pressure crusher, 4°C centrifuge, horizontal shaker (Shanghai Zhichu Instrument Co., Ltd.).
[0053] 2. Experimental steps
[...
Embodiment 2c
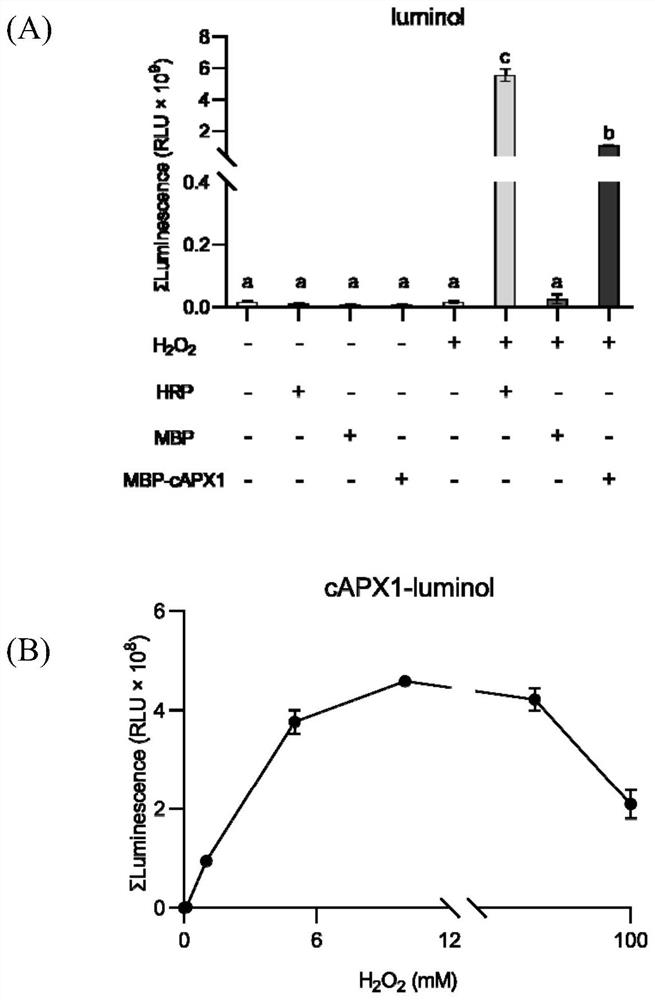
[0072] Example 2 cAPX1 protein catalyzes luminol and H in vitro 2 o 2 Reaction
[0073] The cAPX1 protein purified in Example 1 was used to detect the luminescence intensity after catalyzing luminol.
[0074] 1. Reagent materials and equipment
[0075] 96-well plate (white) (Wohong Bio, Cat # WHB-96);
[0076] Purification of maltose binding protein MBP from Escherichia coli;
[0077] Purify MBP-tagged MBP-cAPX1 recombinant protein from Escherichia coli;
[0078] 10mg / mL HRP (Peroxidase from horseradish, Sigma-Aldrich): Weigh 20mg HRP powder and dissolve in 2mL ddH 2 O, then aliquoted into 1.5mL light-proof centrifuge tubes (50μL per tube) and stored at -20°C as a storage solution;
[0079] 30%H 2 o 2 (Shanghai test, 30% equivalent to 10M after conversion): Dilute it to a final concentration of 100mM when used;
[0080] 100mM luminol (Luminol, Sigma-Aldrich, S-A4685-25G): Weigh 177mg of luminol powder (MW=177.16) and dissolve it in 10mL KOH, then dispense it into 1.5...
Embodiment 3c
[0093] Example 3 cAPX1 is used for luminol to monitor the dynamics of active oxygen generation in plant cells
[0094] In order to test whether cAPX1 can catalyze the interaction of luminol and H 2 o 2 response, we applied lipopolysaccharide LPS to induce plant intracellular H 2 o 2 accumulation.
[0095] Lipopolysaccharide is the main component of the outer membrane of Gram-negative bacteria. After treating the plant leaves, it was observed under a microscope by fluorescent staining that lipopolysaccharide induced the accumulation of intracellular reactive oxygen species, which were mainly located at the periphery of the chloroplast in the cytoplasm.
[0096] Monitoring by luminol chemiluminescence method found that lipopolysaccharide can cause biphasic ROS burst, the first ROS burst rapidly increased within 30min and lasted for a short time; the second ROS burst (later ROS burst) at It started after 3 hours of lipopolysaccharide treatment, reached the maximum value at ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com