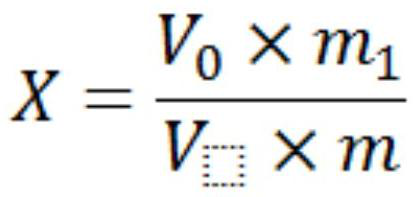Method for measuring iodine content in multi-mineral calcium iodate
A measurement method, the technology of calcium mineral iodate, applied in the direction of color/spectral characteristic measurement, test sample preparation, etc., can solve the problems of unstable data, large difference in iodine content, large error, etc., to achieve accurate measurement results, The effect of wide application range and improved accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Determination of iodine content in calcium iodate by sodium thiosulfate reduction:
[0030] 1. Dissolve 0.2g of a polymine sample with known iodine content in 3ml of nitric acid (1:1 (i.e. double the volume of concentrated nitric acid)), add 0.03 g of solid sodium thiosulfate, and stir thoroughly to make the iodate in the polymine The ions are reduced to iodide ions under acidic conditions, the reduction time is 15 minutes, then filtered and transferred to a 50ml volumetric flask to constant volume, diluted with water to the mark, to be tested.
[0031] 2. Configuration of calcium iodate standard working solution
[0032] After dissolving 0.12 g of the weighed calcium iodate standard sample with 3 ml of nitric acid (1:1), add 0.03 gram of sodium thiosulfate solid, and fully stir to reduce the iodate ions in the polymine to iodide ions under acidic conditions. The reduction time was 15 minutes, then filtered and transferred to a 50ml volumetric flask to constant volume,...
Embodiment 2
[0035] 1. Dissolve 0.155g of polymineral sample with known iodine content in 3ml of hydrochloric acid (1:1), add 0.03g of solid sodium thiosulfate, stir well to reduce the iodate ion in polymineral to iodine under acidic conditions Ions, the reduction time is 15 minutes, then filtered and transferred to a 50ml volumetric flask to constant volume, diluted with water to the mark, to be tested.
[0036] 2. Configuration of calcium iodate standard working solution
[0037] After dissolving 0.108 g of the weighed calcium iodate standard sample with 3 ml of hydrochloric acid (1:1), add 0.03 gram of sodium thiosulfate solid, and fully stir to reduce the iodate ion in polymineral to iodide ion under acidic conditions, The reduction time is 15 minutes, then filtered and transferred to a 50ml volumetric flask to constant volume, diluted with water to the mark, and set aside.
[0038] 3. Accurately pipette 0ml, 0.2ml, 0.4ml, 0.6ml, 0.8ml, 1.2mL of calcium iodate standard working solutio...
Embodiment 3
[0045] 1. After dissolving 0.168g of polymineral sample with known iodine content in 3ml of hydrochloric acid (1:1), add 0.03g of thiourea solid, and stir thoroughly to reduce the iodate ion in polymineral to iodide ion under acidic conditions. The reduction time is 15 minutes, then filtered and transferred to a 50ml volumetric flask to constant volume, diluted with water to the mark, to be tested.
[0046] 2. Configuration of calcium iodate standard working solution
[0047] After dissolving 0.113g of the weighed calcium iodate standard sample with 3ml of hydrochloric acid (1:1), add 0.03g of thiourea solid, and stir fully to reduce the iodate ions in polymineral to iodide ions under acidic conditions. for 15 minutes, then filtered and transferred to a 50ml volumetric flask to constant volume, diluted with water to the mark, and set aside.
[0048] 3. Accurately pipette 0ml, 0.2ml, 0.4ml, 0.6ml, 0.8ml, 1.2mL of calcium iodate standard working solution into a 25mL volumetric ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com