Granzyme B targeting complex, radiopharmaceutical and preparation method and application of granzyme B targeting complex and radiopharmaceutical
A technology for radiopharmaceuticals and complexes, applied in the field of nuclear medicine, can solve the problems of low tumor immunotherapy efficiency, lack of tumor specificity, and less than 30% effective rate, and achieve optimal pharmacokinetic properties and in vivo metabolic stability. , prepare a simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Synthesis of granzyme B targeting complexes.
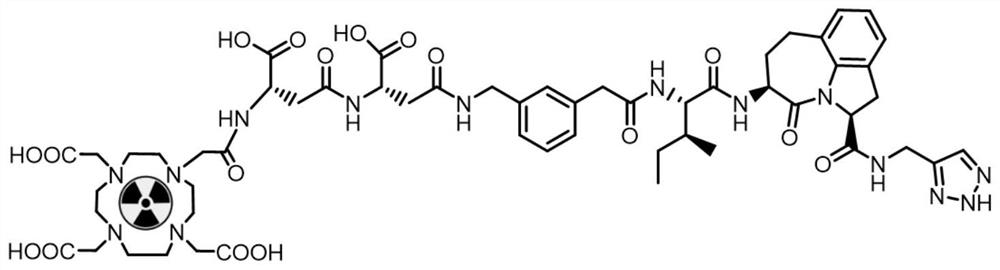
[0064] The granzyme B targeting complex (compound 7) was synthesized according to the following solid-phase synthesis route. Wherein, R is any one of bifunctional chelating groups or derivatives thereof for radionuclide labeling. The bifunctional chelating group is a group formed by bifunctional chelating agents DOTA, NOTA, HYNIC, and MAG2.
[0065]
[0066] Concrete synthetic steps are as follows:
[0067] Reaction conditions: (a) DCM solution of Fmoc-NHS and DIPEA; (b) DMF / DCM solution of 2-chlorotriphenyl chloride resin and DIPEA; (c) DMF solution of 20% piperidine, Fmoc-(2S, 5S) DMF solution of -5-amino-1,2,4,5,6,7-hexahydroazepino[3,2,1-Hi]indole-4-one-2-carboxylic acid, HBTU, HOBt and DIPEA;( d) DMF solution of 20% piperidine, DMF solution of Fmoc-L-isoleucine, HBTU, HOBt and DIPEA; (e) DMF solution of 20% piperidine, Fmoc-(3-aminomethylphenyl) The DMF solution of acetic acid, HBTU, HOBt and EIPEA; (f) the DMF ...
Embodiment 2
[0075] Synthesis of DOTA coupling complex and its 68 Ga, 64 Cu, 111 In, 86 The labeling of any radionuclide in Y is prepared into corresponding radiopharmaceuticals. Specifically include the following steps:
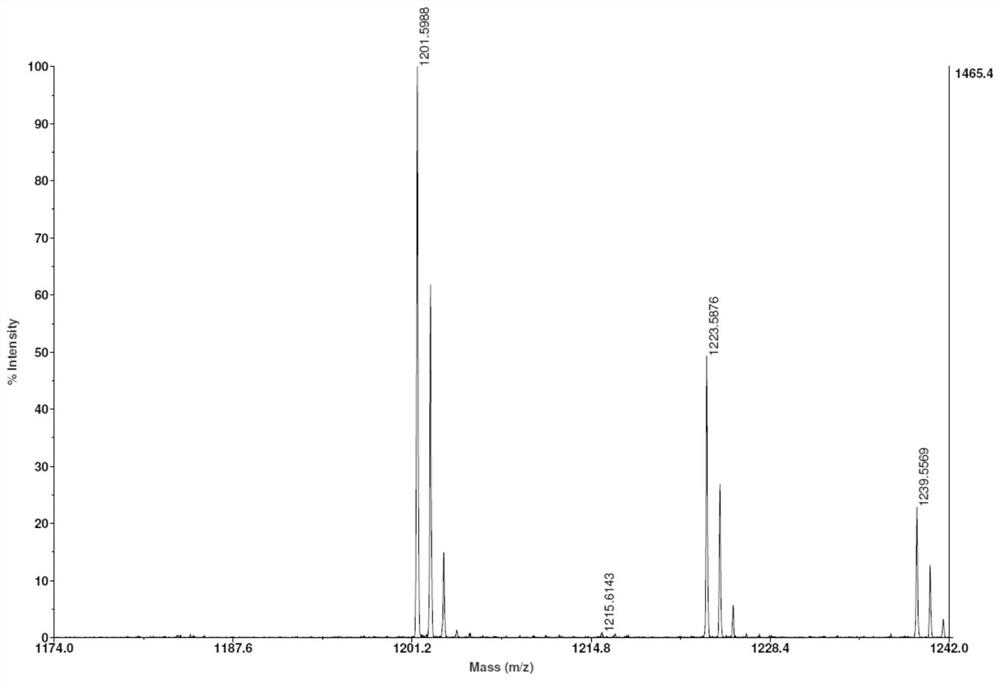
[0076] Take 5 μmol of compound 6, dissolve it in 500 μL of DMSO, and then add 50 μmol of DOTA-NHS and 10 μmol of DIPEA. Mix well and react at room temperature for 2 hours. The crude product is purified by HPLC and freeze-dried to obtain the DOTA-coupled granzyme B targeting complex. For its mass spectrometric characterization, see figure 1 .
[0077] Dissolve 10nmol of DOTA coupling complex in 300μL of 0.1M sodium acetate buffer (pH=5.5); then add 185MBq of 68 GaCl 3 , 64 CuCl 2 , 111 InCl 3 or 86 YCl 3 , reacted at 37°C for 30min, then, the reaction solution was separated and purified by Sep-PakC18 chromatographic column, the purified product was diluted with normal saline and then sterile filtered to obtain the corresponding 68 Ga, 64 Cu, 111 In or 86...
Embodiment 3
[0079] Synthesis of NOTA coupling complex and its 68 Ga, 64 Cu or 18 F Labeling of any radionuclide to prepare corresponding radiopharmaceuticals. Specifically include the following steps:
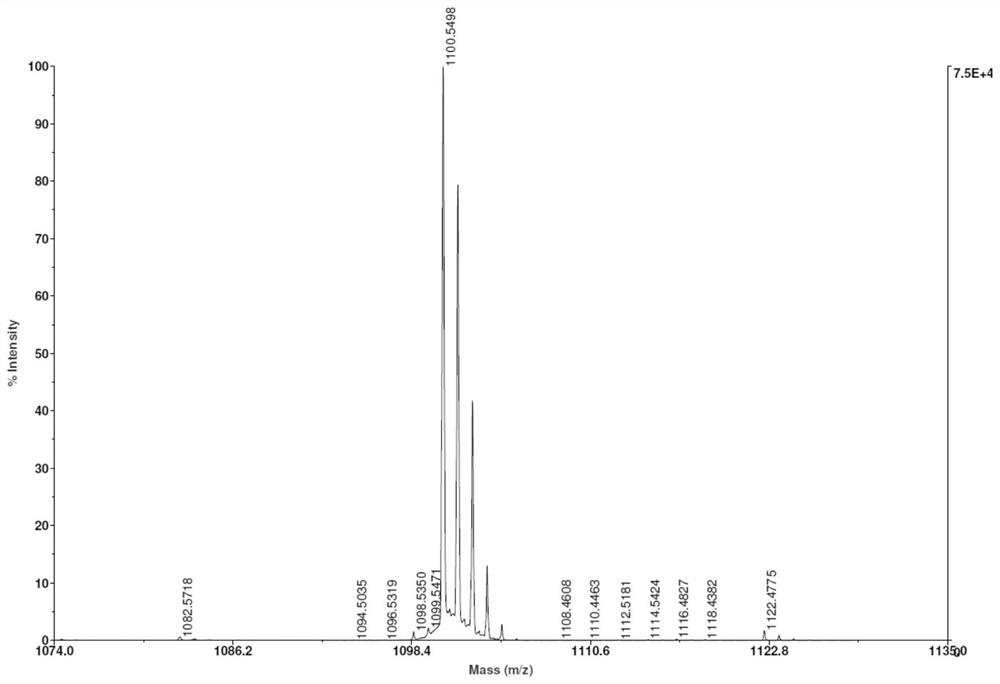
[0080] Take 5 μmol of compound 6, dissolve it in 500 μL of DSMO, then add 50 μmol of NOTA-NHS, and 10 μmol of DIPEA. Mixed and reacted at room temperature for 2 hours, the crude product was purified by HPLC and freeze-dried to obtain the NOTA-coupled granzyme B targeting complex. For its mass spectrometric characterization, see image 3 .
[0081] 68 Ga or 64 Cu radiolabeling: Dissolve 10nmol of NOTA coupling complex in 300μL of 0.1M sodium acetate buffer (pH=5.5); then add 185MBq of 68 GaCl 3 or 64 CuCl 2 , reacted at 37°C for 15 minutes, and after cooling, the reaction solution was separated and purified by Sep-Pak C18 chromatographic column, and the purified product was diluted with normal saline and filtered aseptically to obtain the corresponding 68 Ga or 64 Cu-labeled co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com