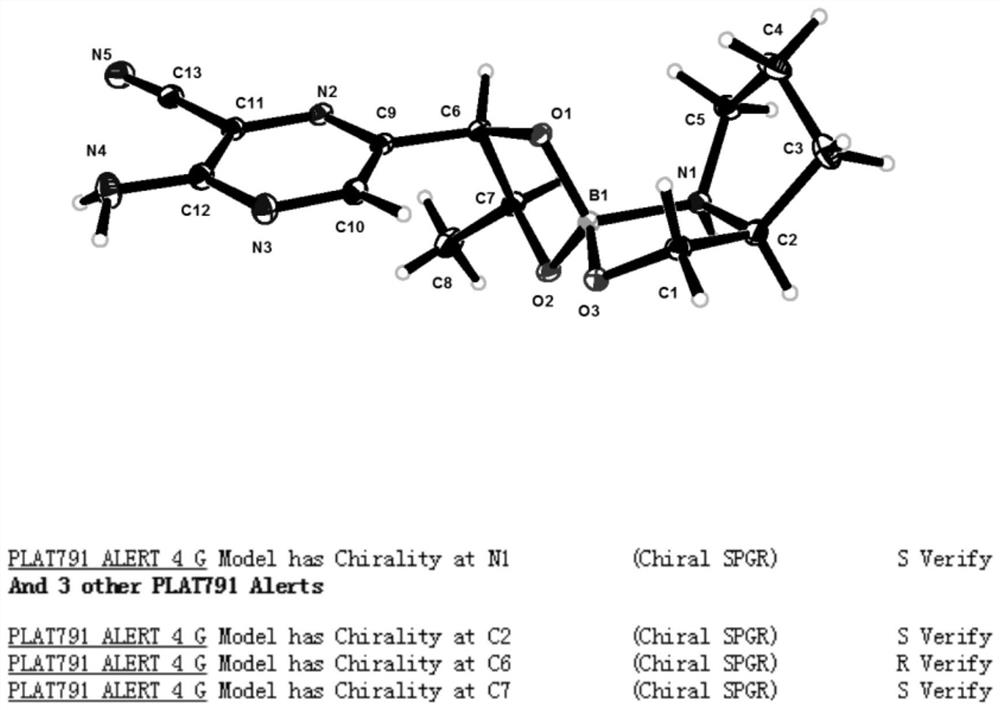
Intermediate for preparing L-red biopterin compound and preparation method thereof
A technology of intermediates and compounds, applied in the field of intermediates and preparations of L-erythro-type biopterin compounds, can solve problems affecting quality, poor selectivity, pollution, etc., and achieve easy storage and transportation, and easy quality control , requiring a wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0106] The present invention also provides the preparation method of above-mentioned intermediate, comprises the following steps:
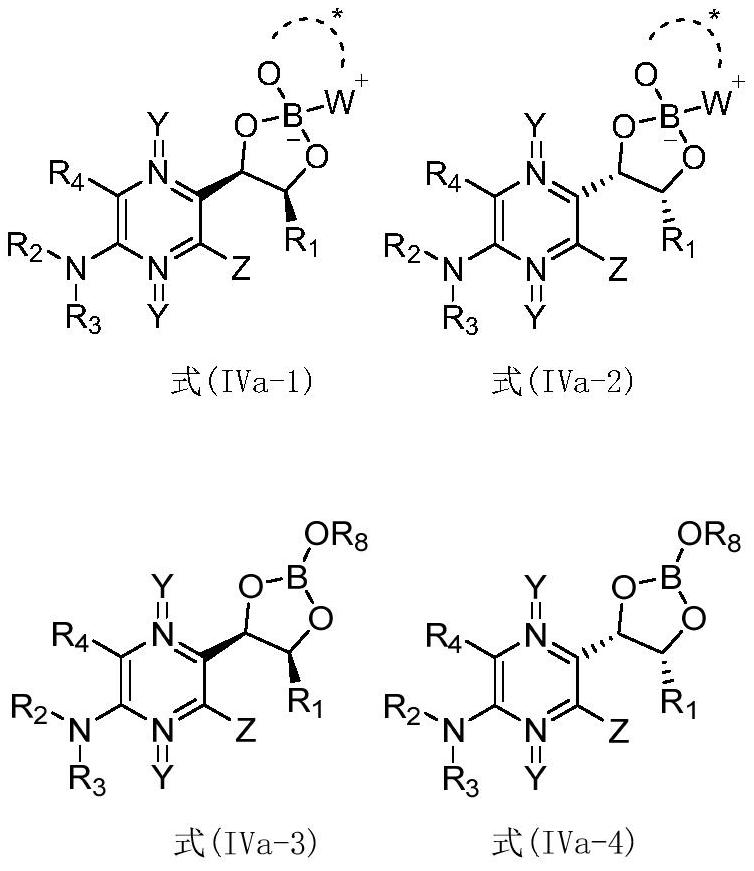
[0107] Mix the compound to be resolved, the first reagent, the second reagent and an aprotic solvent, heat to reflux, and after the reaction is completed, crystallize to obtain the intermediate of the structure shown in formula (IVa-1) or formula (IVa-2); wherein , the compound to be resolved is a mixture of formula (IVa) and formula (IVa');
[0108]
[0109] Understandably, the reaction process of above-mentioned reaction should not be interpreted as limitation of the present invention, and above-mentioned reaction can make to be resolved compound and the first reagent reaction generation formula (IVa-3) and the structure shown in formula (IVa-4) earlier The mixture, and then the mixture of the structures shown in formula (IVa-3) and formula (IVa-4) is reacted with a second reagent to prepare an intermediate of the structure shown in formula (...
Embodiment 1
[0179]
[0180] Take 10g (50mmol) compound 8, CuI 475mg (2.5mmol), PdCl 2 (440mg 2.5mmol), TPP 1.3g (5mmol), TEA (25.3g 250mmol) and propyne 55mL (1M), dissolved in 250mL of acetonitrile, stirred at room temperature for 16h, HPLC detection of raw materials completely converted to products, add 100mLH 2 O washed, separated, the aqueous phase was extracted with 25mL x3 EA, the oil phase was collected, Na 2 SO 4 After drying, column chromatography (EA:Heptane=3:1) obtained compound 6, 7.8 g of yellow crystals, with a yield of 98.7%.
[0181]
[0182] Take 2g (12.5mmol) of compound 6, put it in a hydrogenation kettle, add 20mL 2-MeTHF to dissolve it, add 20mg of Lindlar Pd, replace the H 2 And pressurize 0.2MPa, stir at room temperature, monitor the reaction by HPLC until the raw material just disappears, filter Lindlar Pd, and concentrate, column chromatography (EA:Heptane=1:3) to obtain compound 5a, yellow crystal 1.9g. IR (cm -1 )ν3401, 3202, 2222, 1644, 1492, 1515, 1...
Embodiment 2
[0197] The preparation of 4a and 4b racemate mixture is with embodiment 1;
[0198]
[0199] Dissolve 100mg of the racemic mixture of 4a and 4b in 5mL of acetonitrile, add 117mg of isopropyl borate at the same time, and stir at reflux for 30min. 93.4 mg of L-phenylalaninol was dissolved in acetonitrile and added to the reaction system. Continue to reflux for about 15 minutes, a precipitate occurs, and the precipitate is filtered to obtain 72 mg of product 9a as white crystals with a chemical purity of 99% and a diastereomer ratio (dr) 9a:9b=99.2:0.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com