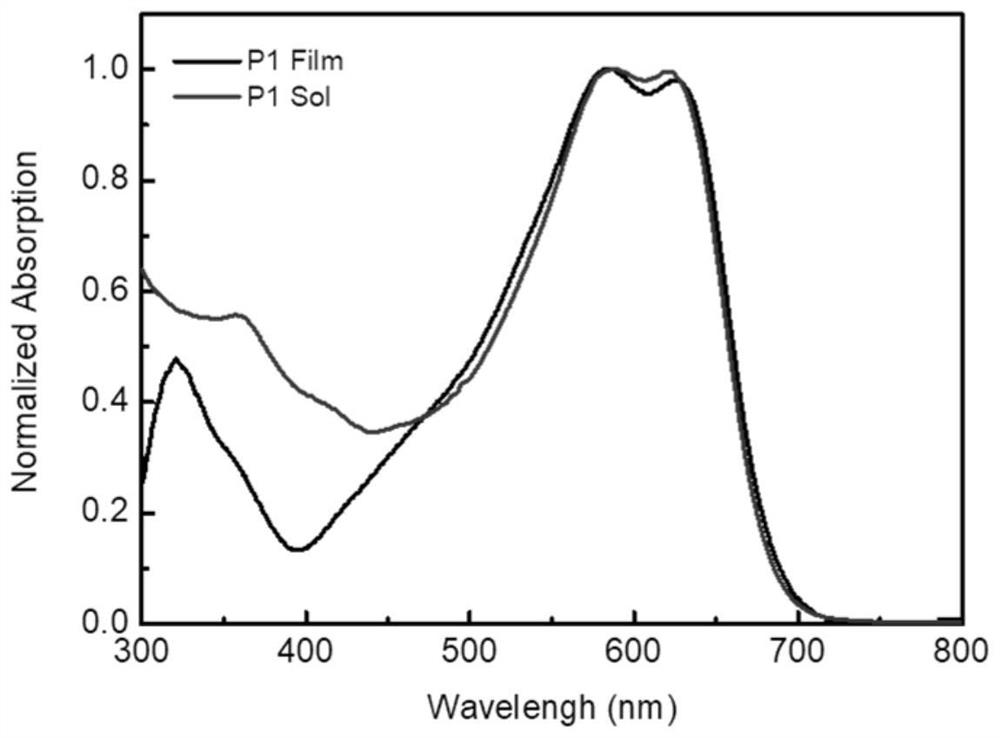
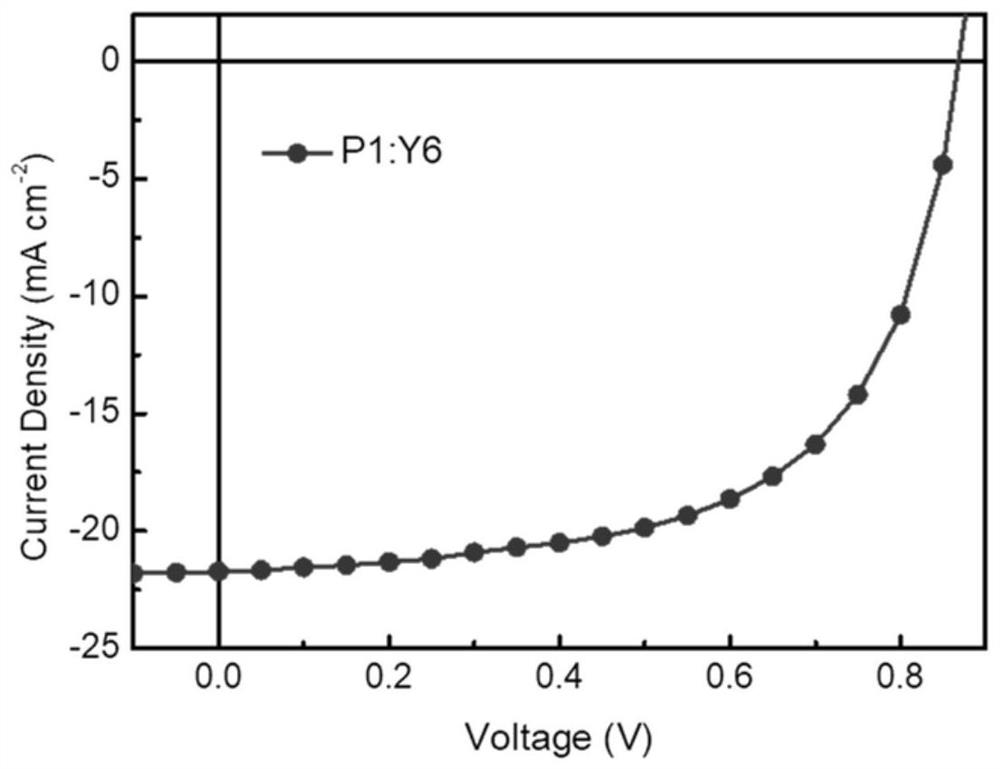
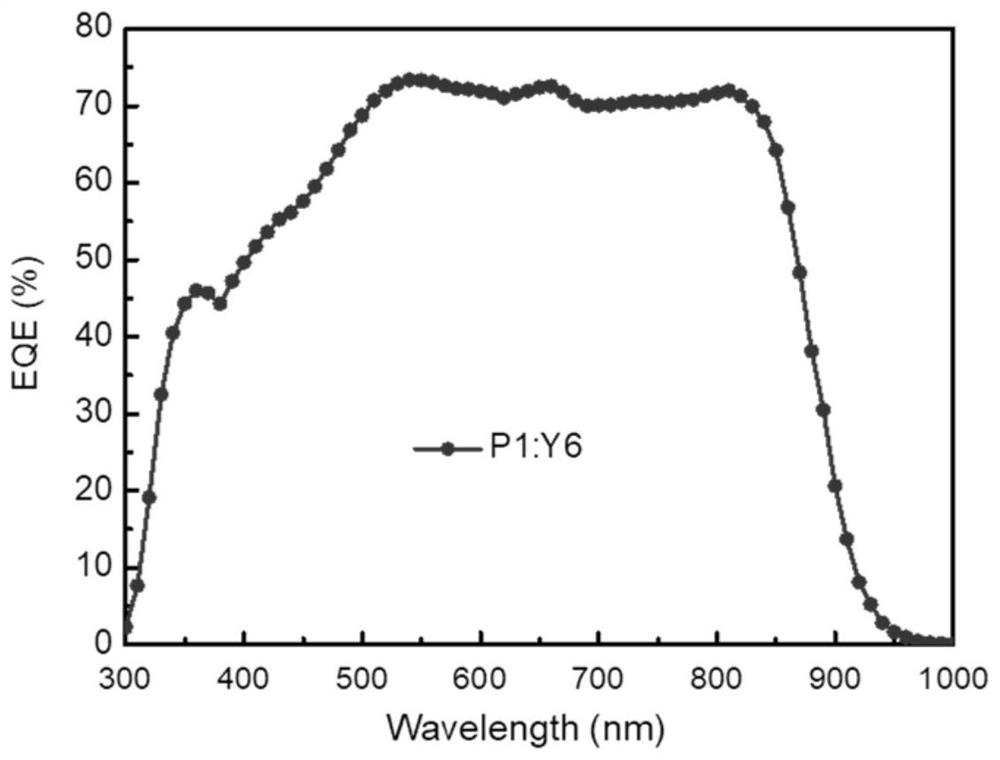
Preparation method and application technology field of polymer donor material containing multi-element aromatic ring thiophenedione
A thiophenedione and polymer technology, applied in the field of solar cells, can solve problems such as energy depletion and environmental pollution, and achieve the effect of high photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The preparation method of the polymer provided by the present invention, polymerizes into formula (I) by monomer formula (II), formula (III),
[0035]
[0036] Wherein W is selected from I, Br or Cl, and V is selected from boronic acid groups, borate ester groups, zinc halide groups or trialkyltin groups;
[0037] The boronic acid group is selected from including but not limited to: 1,3,2-dioxaborolan-2-yl, 4,4,5,5-tetramethyl-1,2,3-dioxane Pentaboran-2-yl or 5,5-dimethyl-1,3,2-dioxaborolan-2-yl;
[0038] The magnesium halide group is selected from including but not limited to: magnesium chloride, magnesium bromide or magnesium iodide; the zinc halide group is preferably: zinc chloride or zinc bromide;
[0039] The trialkyltin group is selected from including but not limited to: trimethyltin, triethyltin or tributyltin.
[0040] If the polycondensation reaction is carried out between a dimagnesium haloarene compound and an arene dihalide, the polymerization is a ty...
Embodiment 1
[0051] Embodiment 1: the synthesis of intermediate 2
[0052]
[0053] At 0°C, potassium tert-butoxide (2.7g, 24.1mmol, 4eq) was added to tetrahydrofuran of fluorene (1.0 g, 6.0mmol) and bromo-n-octane (3.5g, 18.1mmol, 3eq) in several portions. (15mL) solution; after adding, stir at room temperature for 1 hour; add 20mL of water to quench, extract the organic phase with dichloromethane, wash with water to remove impurities, filter and concentrate the organic phase after drying with anhydrous sodium sulfate; petroleum ether As an eluent, the crude product was eluted through a silica gel column to obtain a colorless liquid, i.e. Intermediate 2; 2.0 g of Intermediate 2 was obtained, with a yield of 85%; 1 H NMR analysis was consistent with literature.
Embodiment 2
[0054] Embodiment 2: the synthesis of intermediate 3
[0055]
[0056] At 0°C, aluminum trichloride (1.5g, 11.3mmol, 4.4eq) was added several times to the mixture of intermediate 2 (1.0g, 2.6mmol) and compound C1 (0.94g, 2.6mmol, 1eq). Chloromethane (20 mL) solution; after adding, stir at room temperature for 3 hours; add 20 mL of ice water to quench, extract the organic phase with dichloromethane, wash to remove impurities, and dry the organic phase with anhydrous sodium sulfate, filter and Concentration; petroleum ether and dichloromethane were used as eluents, and the crude product was eluted through a silica gel column to obtain a light yellow liquid, namely intermediate 3; 0.5 g of intermediate 2 was obtained, and the yield was 28%;
[0057] The structural confirmation data is as follows: 1 H NMR (400MHz, CDCl 3 )δ8.59(s,1H),8.29(s,1H),7.90-7.88(d,1H),7.44–7.40(m,3H),2.14–1.96(m,4H), 1.25–0.96(m, 12H), 0.81–0.78(t,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com