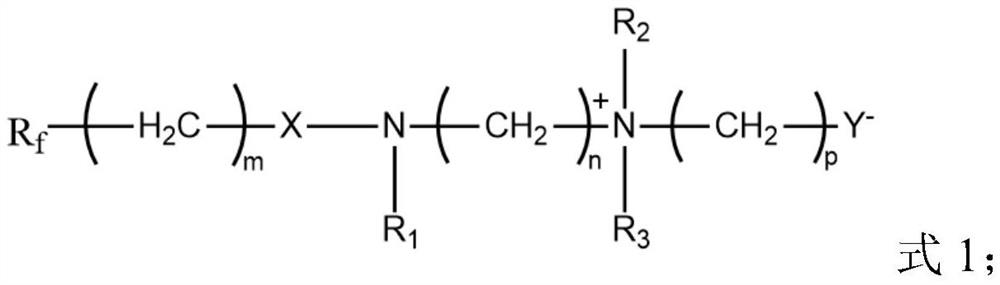
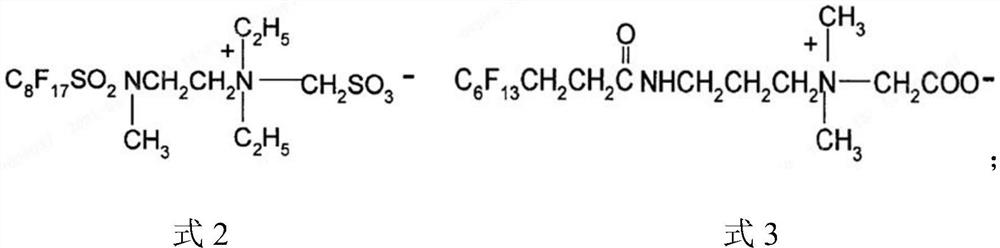
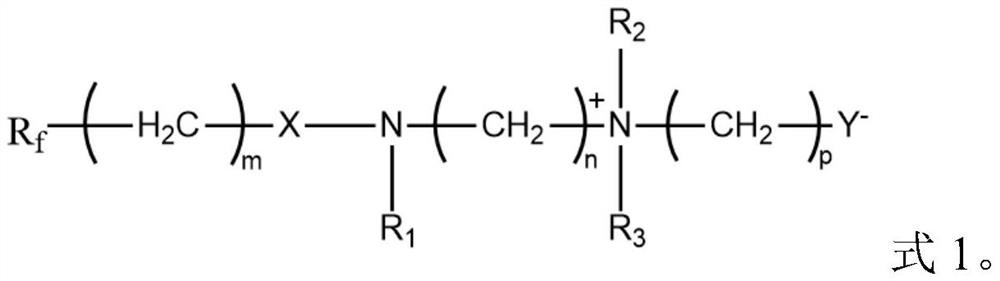
Low-temperature fast-charging electrolyte containing surfactant and application thereof
A surfactant and electrolyte technology, used in circuits, electrical components, secondary batteries, etc., can solve problems such as poor low-temperature performance and fast charging, and achieve the effects of improving cycle performance, reducing low-temperature impedance, and improving wettability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] The present embodiment provides a kind of preparation method of electrolyte:
[0075] The electrolyte lithium salt in the electrolyte is lithium hexafluorophosphate LiPF 6 , the concentration is 1.00mol / L, the solvent system is ethylene carbonate: propylene carbonate: dimethyl carbonate: ethyl methyl carbonate = 30:5:30:35, the additive vinylene carbonate is 2.0%, vinyl sulfate is 0.5%, tris(trimethylsilyl) phosphate 0.3%, perfluoroalkyl betaine 0.1%.
[0076] Wherein, perfluoroalkyl betaine is the compound shown in formula 2, and preparation method is as follows:
[0077] C 8 f 17 SO 2 OCH 3 (51.4g, 0.10mol), CH 3 NHCH 2 CH 2 N(C 2 h 5 ) 2 (15.6g, 0.12mol), catalyst CH 3 ONa (0.054g, 0.001mol) was placed in a reaction flask under nitrogen protection and reacted at 110°C. The by-product methanol was distilled out during the reaction for 1 hour; the excess diamine compound CH was recovered by vacuum distillation. 3 NHCH 2 CH 2 N(C 2 h 5 ) 2 , to obtain ...
Embodiment 2
[0080] The present embodiment provides a kind of preparation method of electrolyte:
[0081] The electrolyte lithium salt in the electrolyte is lithium hexafluorophosphate LiPF 6 , the concentration is 1.00mol / L, the solvent system is ethylene carbonate: propylene carbonate: dimethyl carbonate: ethyl methyl carbonate = 30:5:30:35, the additive vinylene carbonate is 1.5%, vinyl sulfate is 0.5%, tris(trimethylsilyl) phosphate 0.5%, perfluoroalkyl betaine 0.3%.
[0082] Wherein, perfluoroalkyl betaine is the compound shown in formula 2, and preparation method is as follows:
[0083] C 8 f 17 SO 2 OCH 3 (51.4g, 0.10mol), CH 3 NHCH 2 CH 2 N(C 2 h 5 ) 2 (15.6g, 0.12mol), catalyst CH 3 ONa (0.054g, 0.001mol) was placed in a reaction flask, protected by nitrogen, and reacted at 100°C. The by-product methanol was distilled out during the reaction for 1 hour; the excess diamine compound CH was recovered by vacuum distillation. 3 NHCH 2 CH 2 N(C 2 h 5 ) 2 , to obtain th...
Embodiment 3
[0086] The electrolyte lithium salt in the electrolyte is lithium hexafluorophosphate LiPF 6 , the concentration is 1.00mol / L, the solvent system is ethylene carbonate: propylene carbonate: dimethyl carbonate: ethyl methyl carbonate = 30:5:30:35, the additive vinylene carbonate is 3.0%, vinyl sulfate is 0.1%, tris(trimethylsilyl) phosphate 0.2%, perfluoroalkyl betaine 0.5%.
[0087] Wherein, perfluoroalkyl betaine is a compound shown in formula 3, and the preparation method is as follows:
[0088] C 6 f 13 CH 2 CH 2 CO 2 C 2 h 5 (42.0g, 0.10mol), NH 2 CH 2 CH 2 CH 2 N(CH 3 ) 2 (12.2g, 0.12mol), Catalyst C 2 h 5 ONa (0.068g, 0.001mol) was placed in a reaction flask, protected by nitrogen, and reacted at 120°C. The by-product methanol was distilled out during the reaction for 1 hour; the excess diamine compound NH was recovered by distillation under reduced pressure. 2 CH 2 CH 2 CH 2 N(CH 3 ) 2 , to obtain the intermediate product perfluoroalkyl tertiary ami...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com