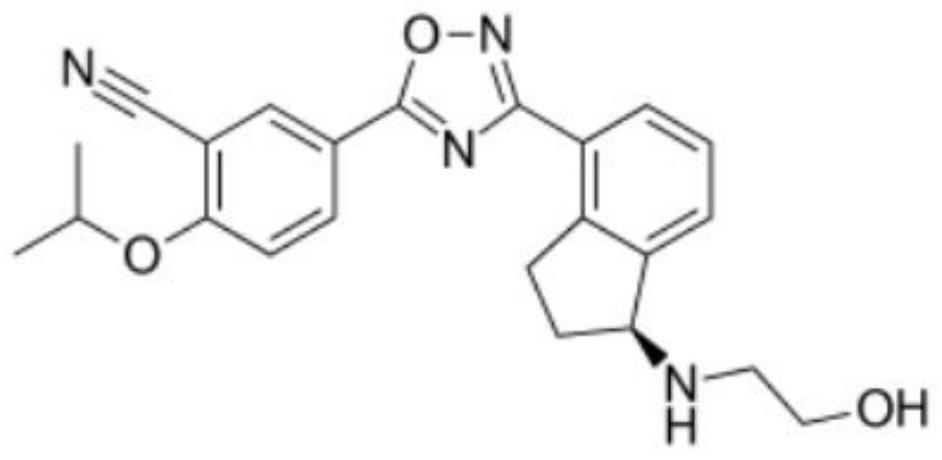
Application of Ozanimod in preparation of medicine for treating muscular dystrophy
A technology for muscular dystrophy and muscular dystrophy, applied in the field of medicine, can solve problems such as adverse reactions of patients, gene therapy methods are in the research stage, etc., and achieve the effect of reducing inflammation, improving muscle weakness and/or muscle atrophy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1- Construction of animal model:
[0021] mdx mice are DMD gene defects, leading to the absence of dystrophin protein, a well-recognized model mouse for the study of Duchenne / Bein muscular dystrophy. Compared with the wild control group, mdx mice had abnormal muscle tissue morphology; markers of muscle degeneration, serum levels of creatine kinase and lactate dehydrogenase increased; a large number of macrophages accumulated in muscle tissue, accompanied by fibrosis and The occurrence of necrosis. The experimental animals used in the present invention are mdx transgenic mice named (C57BL / 10ScSnJNju-DmdDMD / Nju), purchased from Nanjing Jicui Yaokang Company.
[0022] 2- Animal grouping and administration
[0023] The present invention administers ozanimod to mdx mice by intragastric administration, at a dose of 1 mg / kg / day, and feeds them for 42 days. The muscle grip strength of the mice was measured on the 40th day of administration, the rod hanging time of the mice w...
Embodiment 2
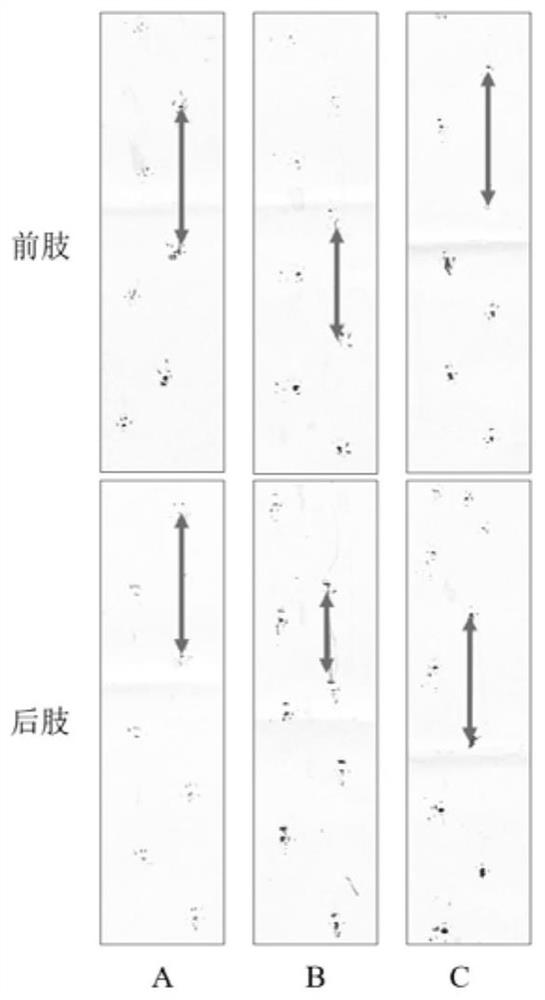
[0042] Gait analysis: the gait analysis and statistics of the mdx mice after the 41st day of administration in Example 1 were carried out using the ink method. The stride length and stride width of the front and hind limbs of mice in each group were recorded respectively. The average value of each test was taken 3 times, and the results are shown in Table 4.
[0043] Table 4 Statistical results of mdx mouse gait experiment
[0044] group normal group DMD model group ozanimod treatment group Forelimb stride length (cm) 6.5±0.5 5.0±0.7 *
6.1±0.2 #
Hindlimb stride length (cm) 6.9±0.5 5.3±0.6 *
6.2±0.5 #
Forelimb stride width (cm) 2.0±0.3 1.7±0.1 *
1.8±0.1 #
Hind leg width (cm) 2.6±0.3 2.9±0.5 *
2.5±0.2 #
[0045] * P# P<0.05 compared with DMD model group.
[0046] The results showed that the forelimb step width of the mice in the DMD model group became narrower, the hindlimb step length became shorter ...
Embodiment 3
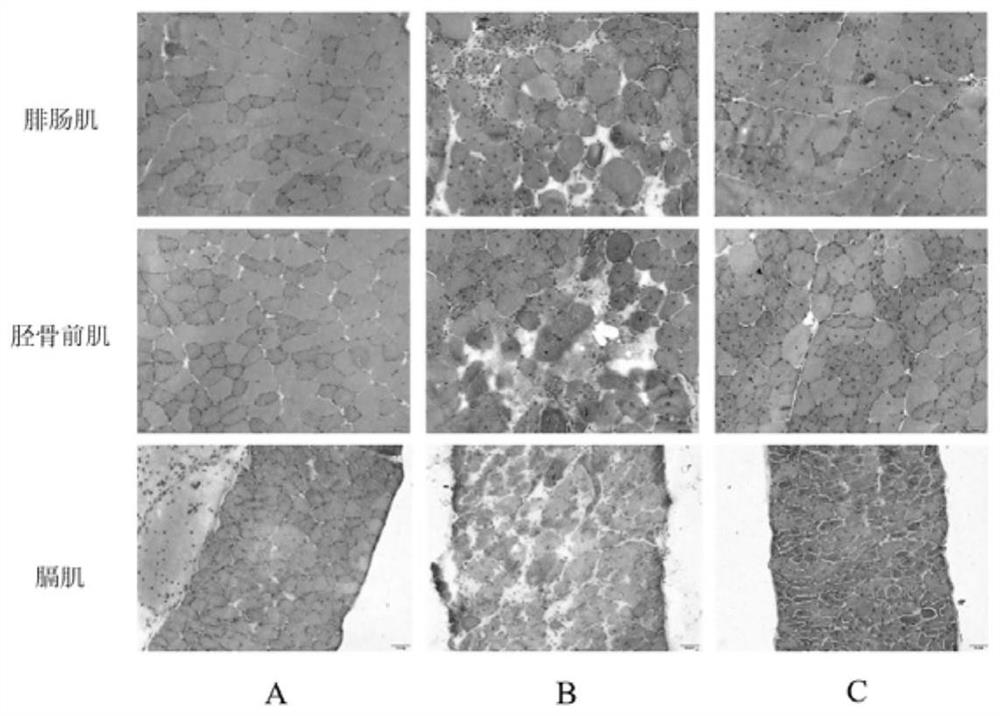
[0048] After 42 days of administration, the mdx mice in Example 1 were euthanized on the 43rd day after overnight fasting for 12 hours, blood was taken from the inguinal vein, centrifuged at 3500 rpm for 10 minutes, and the upper serum was taken to measure serum creatine kinase (CK) and lactic acid Dehydrogenase (LDH) content. Then the gastrocnemius, tibialis anterior muscle, and diaphragm were dissected and quickly frozen in liquid nitrogen, and transferred to a minus 80 refrigerator for later use. The content of serum CK and LDH and the weight of the first few muscles of the tibia are shown in Table 5.
[0049] Table 5 mdx mouse tibialis anterior muscle weight
[0050] group normal group DMD model group ozanimod treatment group CK(U / L) 1942.5±550.3 18900.0±7145.6 *
16524.0±4122.5 #
LDH(U / L) 542.5±196.8 5824.3±2019.8 *
2634.3±600.3 Tibialis anterior muscle weight (mg) 52.2±3.9 63.3±6.8 *
55.3±6.5 #
[0051] * P# P<...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com