Method for inducing pluripotent stem cells to differentiate into melanocytes in serum-free culture solution
A serum-free culture medium, pluripotent stem cell technology, applied in the field of cell engineering and cell biology, can solve the problem of low differentiation efficiency, and achieve the effect of high differentiation efficiency and short time-consuming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Differentiation of the H9 human embryonic stem cell line into epidermal melanocytes:
[0066] 1. Melanocyte differentiation:
[0067] 1. The configuration of culture medium:
[0068] Medium No. 1: Add 10-50% KnockOut produced by Gibco to 500mL DMEM / F-12 (1:1) medium TM Serum Replacement, then add 4-20ng / mL recombinant human fibroblast growth factor 2 (FGF-2) and 2-50mM high-purity β-mercaptoethanol.
[0069] Medium No. 2: Add 1-10g / L human transferrin, 50-200mg / L recombinant human insulin, and 0.063-0.63mg / L corpus luteum to 500mL DMEM / F-12 (1:1) medium Ketones, 100-1600mg / L putrescine and 0.1-1mg / L sodium selenite.
[0070] DiffMel medium: based on Ham's F12 Medium, add 4-20ng / mL recombinant human FGF-2, 0.1-10ng / ml recombinant human stem cell factor (SCF), 0.1-200nM human endothelin 3 (EDN3), 0.1-10 ng / mL bone morphogenetic protein 4 (BMP4), 1-10% bovine serum albumin (BSA), 1-200 uM ascorbic acid, 1-10 uM CHIR99021, 0.1-1 mM dibutyryl cyclic adenosine (dbcAMP),...
Embodiment 2
[0115] Differentiation of human induced pluripotent stem cells into epidermal melanocytes:
[0116] Human induced pluripotent stem cells were induced to differentiate into epidermal melanocytes according to the melanocyte differentiation method described in Example 1.
[0117] Characterization of differentiated melanocytes:
[0118] 1. Cell morphology:
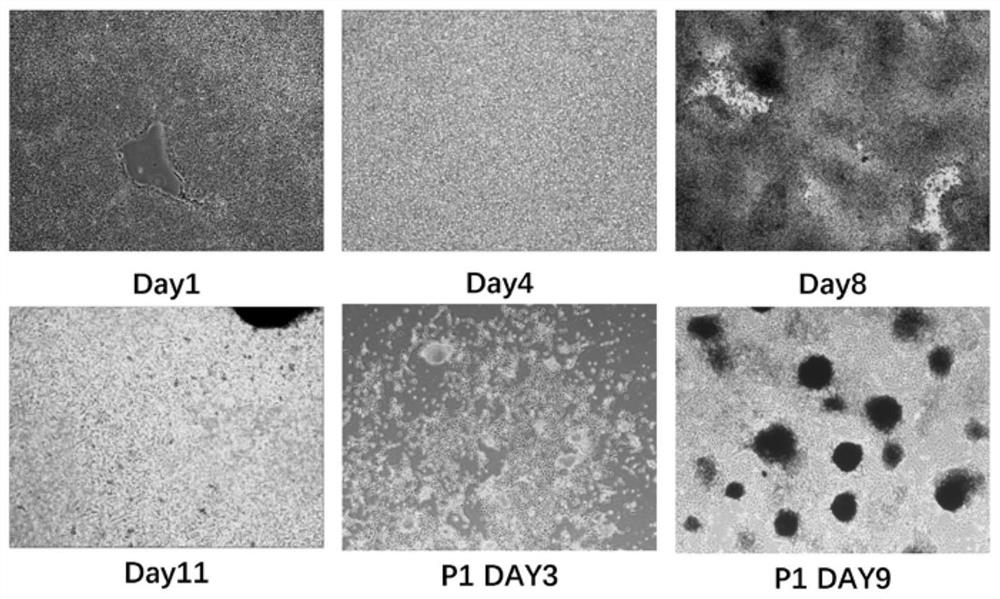
[0119] Such as Figure 11 As shown, the changes in cell morphology throughout the differentiation process are demonstrated.
[0120] Changes in cell morphology during differentiation of human induced pluripotent stem cells into melanocytes. The first row of pictures from left to right shows the cell morphology on day 1, day 3, and day 8 of differentiation, and the second row of pictures shows from left to right day 11 of differentiation, day 3 after passage, and passage The cell morphology on the 7th day after subculture shows that obvious melanocytes have appeared on the 7th day after subculture.
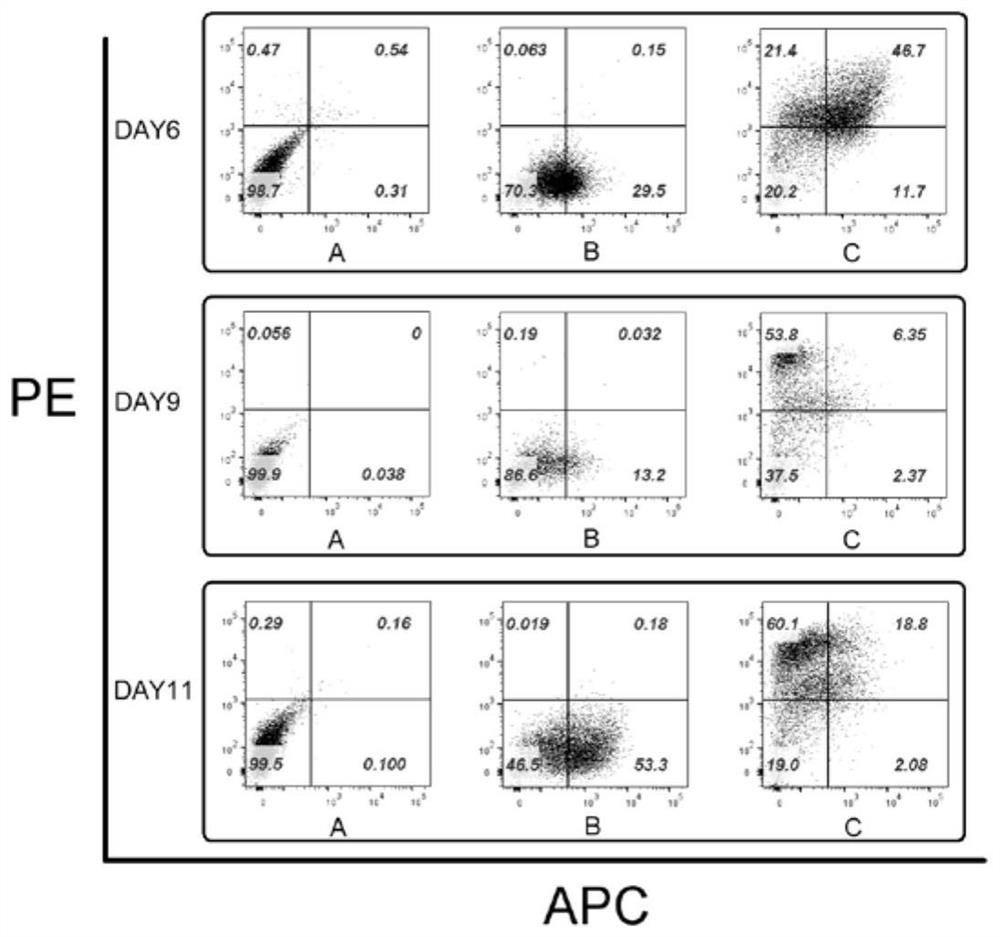
[0121] Such as Figur...
Embodiment 3
[0126] Comparison to Other Melanocyte Cell Differentiation Methods
[0127] using equivalent Basement Membrane Matrix, 3x10 in LDEV-free coated cell culture dishes 6 H9 embryonic stem cells and Essential 8 produced by Gibco TM Medium cultured to day 3. Subsequently, the human embryonic stem cells were differentiated into melanocytes by following the melanocyte differentiation method provided in Example 1, and the embryonic stem cells were differentiated into melanocytes by using other two reported differentiation methods at the same time. On the 11th day, the difference in the expression of melanocyte-related genes among the three groups of cells was detected by Q-PCR, and embryonic stem cells and primary human melanocytes were used as controls. The results are as follows Figure 15 shown. From Figure 15 It can be seen from the figure that the levels of melanocyte-related PAX3, SOX10, MITF, and TYR genes expressed by the cells differentiated by the method in Example 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com