Method for realizing controllable free radical polymerization in air atmosphere and application thereof
A free radical and polymerization reaction technology, applied in the field of polymer synthesis, can solve the problems of long polymerization induction period, cumbersome experimental operation, limited oxygen tolerance and controllable free radical polymerization application field, and achieve high-throughput optimization of polymerization conditions, High monomer conversion rate, fast and efficient polymerization reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] (1) Preparation of the mixed solution: dissolving the functional monomer, the reversible addition-fragmentation chain transfer reagent and the alkylboramine complex in a solvent and mixing to obtain the mixed solution;
[0051] (2) Polymerization reaction: heating the mixed liquid obtained in step (1), the alkyl boron amine complex releases the alkyl boron compound, the alkyl boron compound autoxidizes to generate an alkyl radical, and the alkyl free radical initiates functional Monomer polymerization forms growth free radicals, and the growth free radicals undergo degeneration transfer with reversible addition-fragmentation chain transfer reagents to realize controllable free radical polymerization;
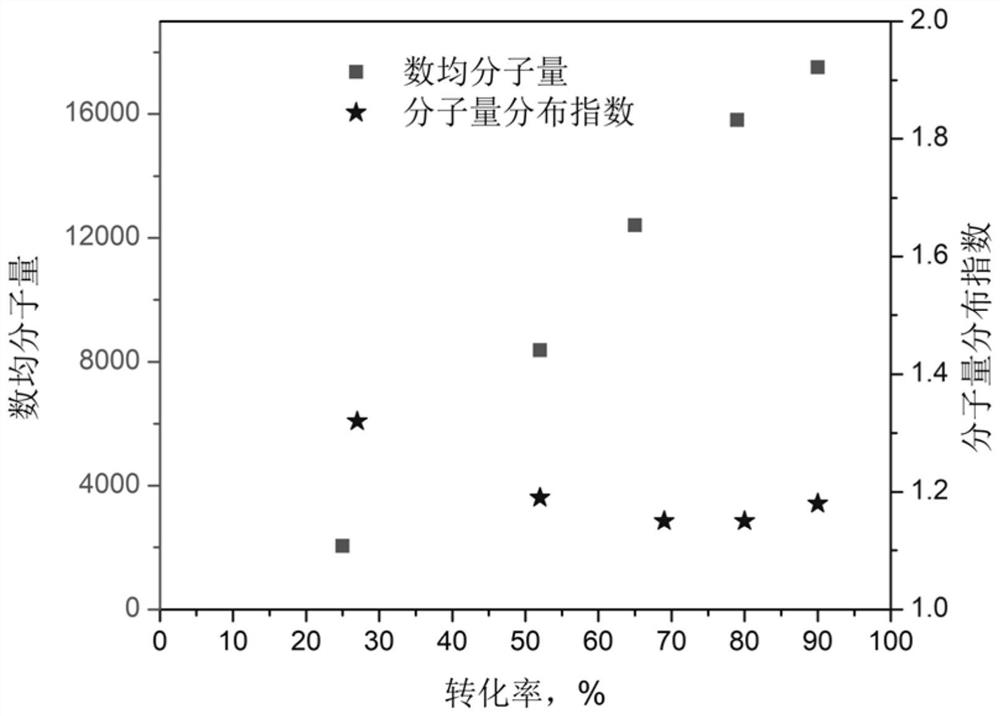
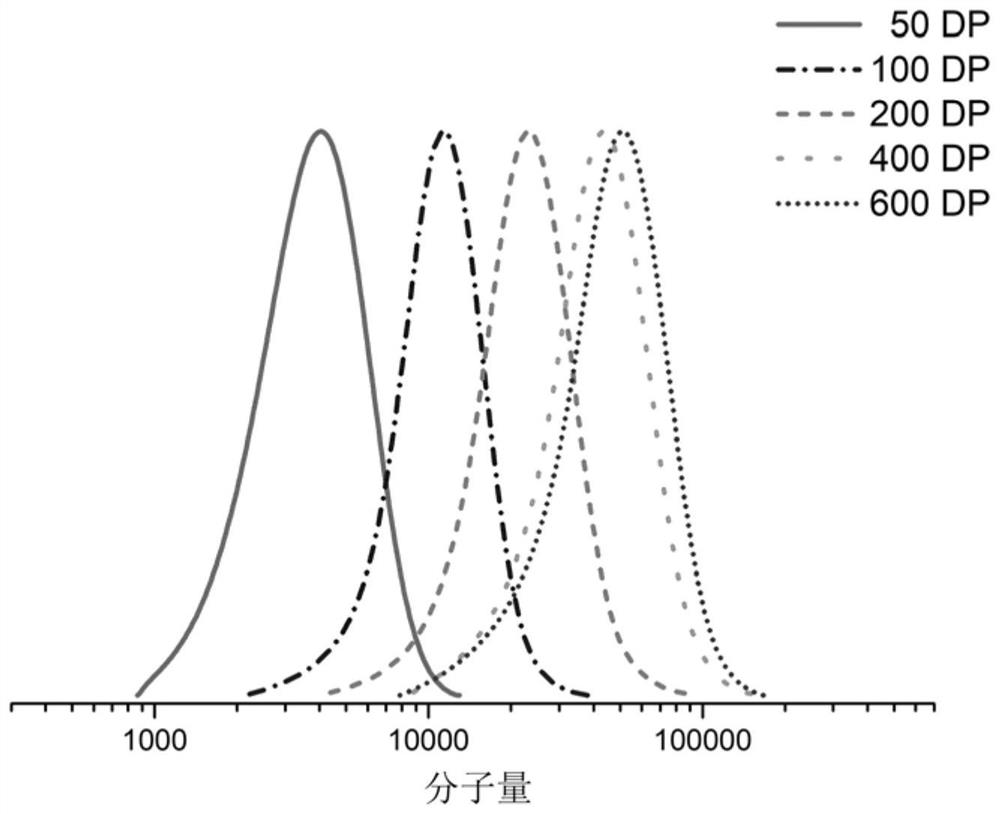
[0052] (3) After the polymerization reaction is finished, the temperature is lowered to obtain a polymer with controllable molecular weight, narrow molecular weight distribution and active end groups;
[0053] The whole process is completely exposed to the air.
[0054] ...
Embodiment 1
[0074] This embodiment provides triethylboron-4-dimethylaminopyridine complex (Et 3 B-DMAP) preparation, chemical equation is as follows:
[0075]
[0076] In a 50 mL reaction flask, under N 2 Add triethylboron tetrahydrofuran solution (10 mL, 1 mol / L) under the protection of . The above reaction flask was placed in an ice-water bath, and then 2 Slowly add 1.22 g of 4-dimethylaminopyridine (10 mmol) in batches in the atmosphere, continue to react in the ice-water bath for 10 minutes, then remove the ice-water bath, and react at room temperature for 1 hour. After the reaction was completed, the solvent tetrahydrofuran was removed by rotary evaporation under reduced pressure to obtain 2.02 g of white crystals with a yield of 92%. 1 H-NMR nuclear magnetic spectrum analysis is triethylboron-4-dimethylaminopyridine complex (Et 3 B-DMAP).
Embodiment 2
[0078] This embodiment provides triethylboron-4-methoxypyridine complex (Et 3 B-4MeO-Py) preparation, chemical equation is as follows:
[0079]
[0080] In a 50 mL reaction flask, under N 2 Add triethylboron tetrahydrofuran solution (10 mL, 1 mol / L) under the protection of . The above reaction flask was placed in an ice-water bath, and then 2 In the atmosphere, 1.09 g of 4-methoxypyridine (10 mmol) was slowly added dropwise, and the reaction was continued in the ice-water bath for 10 minutes, then the ice-water bath was removed, and the reaction was carried out at room temperature for 1 hour. After the reaction was completed, the solvent tetrahydrofuran was removed by rotary evaporation under reduced pressure to obtain 1.97 g of a colorless oily liquid with a yield of 95%. 1 H-NMR nuclear magnetic spectrum analysis is triethylboron-4-methoxypyridine complex (Et 3 B-4MeO-Py).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com