Recombinant antigen protein rP44-60 for detecting granulocytoplasmosis and kit containing antigen
A technology of recombinant antigen protein and anaplasma, applied in the field of immunology, can solve the problems of strengthening the detection method of granulocytic anaplasmosis, lack of high sensitivity and high specificity, difficulty in antibody detection, etc., and achieves broad market prospects and application value. The effect of shortening the color time and reducing the missed diagnosis rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] A method for preparing recombinant antigen protein rP44-60 for detection of granulocytic anaplasmosis, comprising the following steps:
[0060] 1) Using bioinformatics technology, based on the existing p44 polygenome sequence as the basic template, the original proteome was synthesized, and then the original proteome was sent to Gene Script for sequencing to confirm that the above original proteome contained the rP44-60 target gene sequence.
[0061] 2) Using the RNA extraction kit (QIAGEN), the RNA extracted from the cultured cells of the susceptible THP-1 was used as a template, and reverse-transcribed into cDNA according to the instructions of the reverse transcription kit. Primers were designed according to the target gene sequence, followed by RT-PCR amplification, and the amplified product was recovered by agarose gel electrophoresis (TaKaRa's DNA recovery and purification kit) to obtain the recovered product. The above reverse transcription conditions are: 37°C, ...
Embodiment 2
[0070] This embodiment provides a kit based on a double-antigen sandwich colloidal gold immunochromatography method, which includes the recombinant antigen protein rP44-60 for the detection of granulocytic anaplasmosis.
[0071] Specifically, the preparation method of the colloidal gold immunochromatography test strip for the kit is as follows:
[0072] 1) Using the colloidal gold probe as a tracer marker, the colloidal gold probe solution is prepared, and then the recombinant antigen protein rP44-60 colloidal gold complex and the mouse IgG colloidal gold complex are prepared respectively.
[0073] 2) Mix the above-mentioned recombinant antigen protein rP44-60 colloidal gold complex and mouse IgG colloidal gold complex, resuspend with 0.1mmol / L Tris solution containing 5% sucrose, 5% BSA, and then adjust the resuspension to OD530 After reaching 2, soak the bonding pad and dry it at 37°C for 4 hours to obtain a gold standard pad.
[0074] 3) Use the recombinant antigenic prote...
experiment example 1
[0087] Antigenicity test
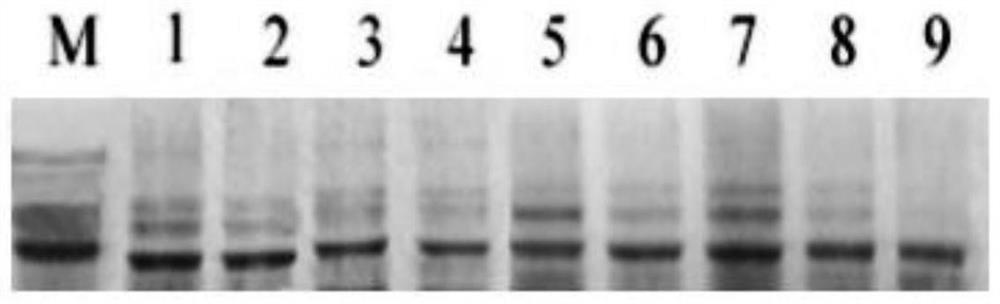
[0088] Experimental method: The recombinant antigen protein rP44-60 prepared in Example 1 was used as an antigen, and the serum of animals infected with Anaplasma phagocytophilum and positive patients was used to detect its antigenicity by WB technology, and its feasibility as an antigen was evaluated. Among the animals, there were 3 positive sera of mice, 3 positive serums of sheep, and 3 positive patient sera. The secondary antibody used Goat Anti-Human IgG Alkaline phosphatase (Invitrogen). First, the recombinant antigen protein rP44-60 was loaded on the SDA-PAGE gel, the protein was transferred to the nitrocellulose membrane, the membrane was cut into strips, blocked for 30min, incubated with the primary antibody positive serum (1:2000), washed with PBS for 3 times, incubated with secondary antibody (1:5000), washed 4 times with PBS, and developed. The result is as figure 1 shown.
[0089] figure 1 It is the result of WB technology detection...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com