Block copolymer, block copolymer drug-loaded micelle as well as preparation method and application of block copolymer drug-loaded micelle
A technology of block copolymer and drug-loaded micelle, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
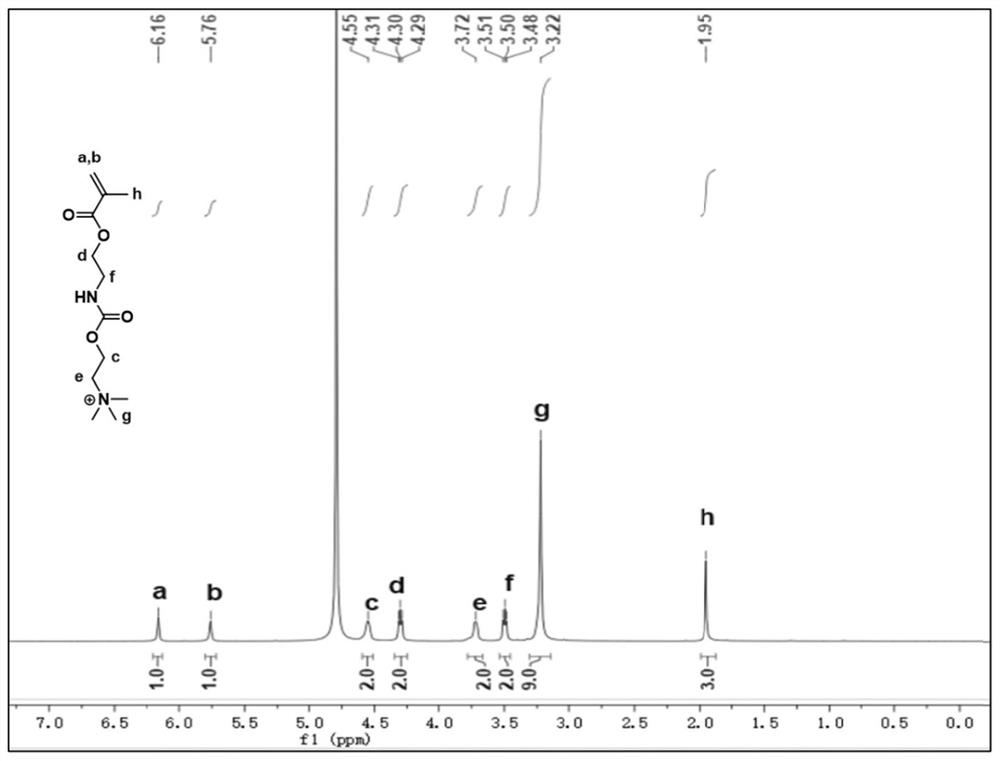
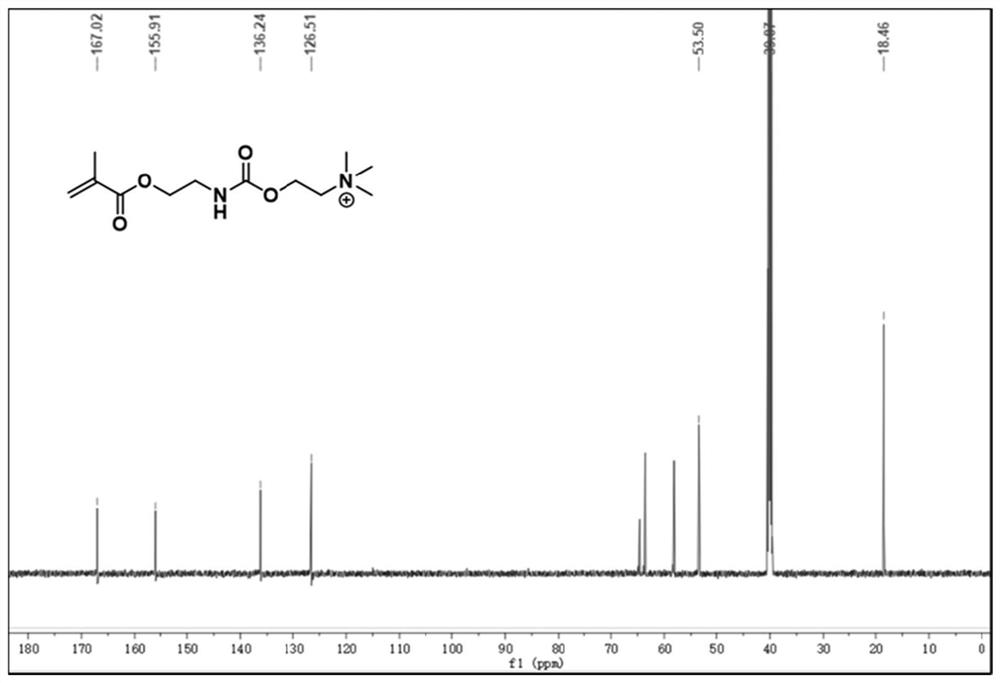
[0060] Embodiment 1 provides a kind of block copolymer, the structural formula of described block copolymer is as follows, and preparation method comprises the following steps:
[0061]
[0062] 1) The preparation of TAMA, the synthetic route is as follows:
[0063]
[0064] S1. Dissolve 1.8g of N,N-dimethylethanolamine (20.0mmol) in 40mL of anhydrous tetrahydrofuran, then add a catalytic amount of dibutyltin dilaurate (40μL), stir for 15 minutes, and slowly add 3.8g of methyl Isocyanoethyl acrylate (24.0 mmol). After stirring at room temperature for 4 h, the reaction mixture was concentrated under reduced pressure, separated and purified through a basic alumina column to obtain 4.2 g of a colorless oily product (yield: 85%).
[0065] S2. Dissolve the product of step S1 (4.2g, 17.2mmol) in anhydrous tetrahydrofuran (30mL), slowly add 2.7g methyl iodide (18.9mmol) dropwise after ice bath for 15 minutes, stir for 30 minutes, and continue to stir at room temperature for 2h...
Embodiment 2
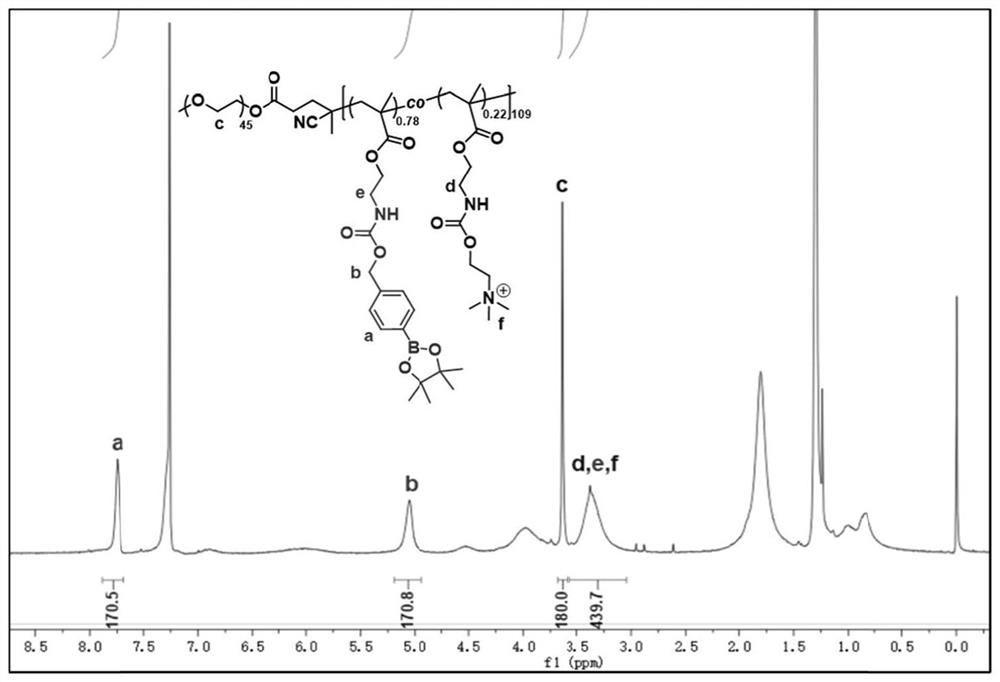
[0072] This embodiment 2 provides a new block copolymer, the difference from embodiment 1 is that the monomers are different, the structural formula is as follows, and the preparation method is as follows:
[0073]
[0074] 83mg benzylacrylamide (0.51mmol), 200mg TAMA (0.51mmol), 238mg polyethylene glycol methyl ether acrylate (Mw: 300) (0.68mmol) and 1.5mg azobisisobutyronitrile (0.009mmol), 3.757 mg of 2-cyanopropyl-2-ylbenzodisulfide (0.017 mmol) was dissolved in 600 μL of N,N-dimethylformamide, then transferred to a polymerization tube, degassed by three freeze-thaw cycles, and finally in vacuo Lower seal. The polymerization tube was then immersed in a preheated 65°C oil bath. After stirring for 10 h, the polymerization tube was placed in liquid nitrogen for a while, and the lid was opened to terminate the polymerization. The reaction mixture was precipitated in 100 mL of ether, then the precipitate was dissolved in a small amount of dimethylformamide and precipitated...
Embodiment 3
[0076] Example 3 provides a block copolymer drug-loaded micelle, which includes the block copolymer prepared in Example 1 and an active pharmaceutical ingredient, where the active pharmaceutical ingredient is camptothecin. The preparation method of described block copolymer drug-loaded micelle comprises the steps:
[0077] The 5mg PEG prepared by embodiment 1 45 -b-P(QPBA 0.78 -co-TAMA 0.22 ) 109 Co-dissolve with camptothecin in 1 mL of a suitable organic solvent (dioxane), quickly add to vigorously stirred PBS (9 mL, 10 mM, pH 7.4), continue stirring for 7 minutes, transfer the sample to a dialysis bag, and Water dialysis to remove organic solvents and unloaded drug.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com