Drug sustained-release controllable polyether-ether-ketone implant and preparation method thereof
A polyetheretherketone and implant technology, which is used in drug delivery, pharmaceutical formulations, joint implants, etc., can solve the problems of easy detachment of the coating, too fast slow release time, uncontrollable porosity of pore-making technology, etc. , to achieve the effects of good biocompatibility, guaranteed mechanical properties, good interlayer bonding and printing accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
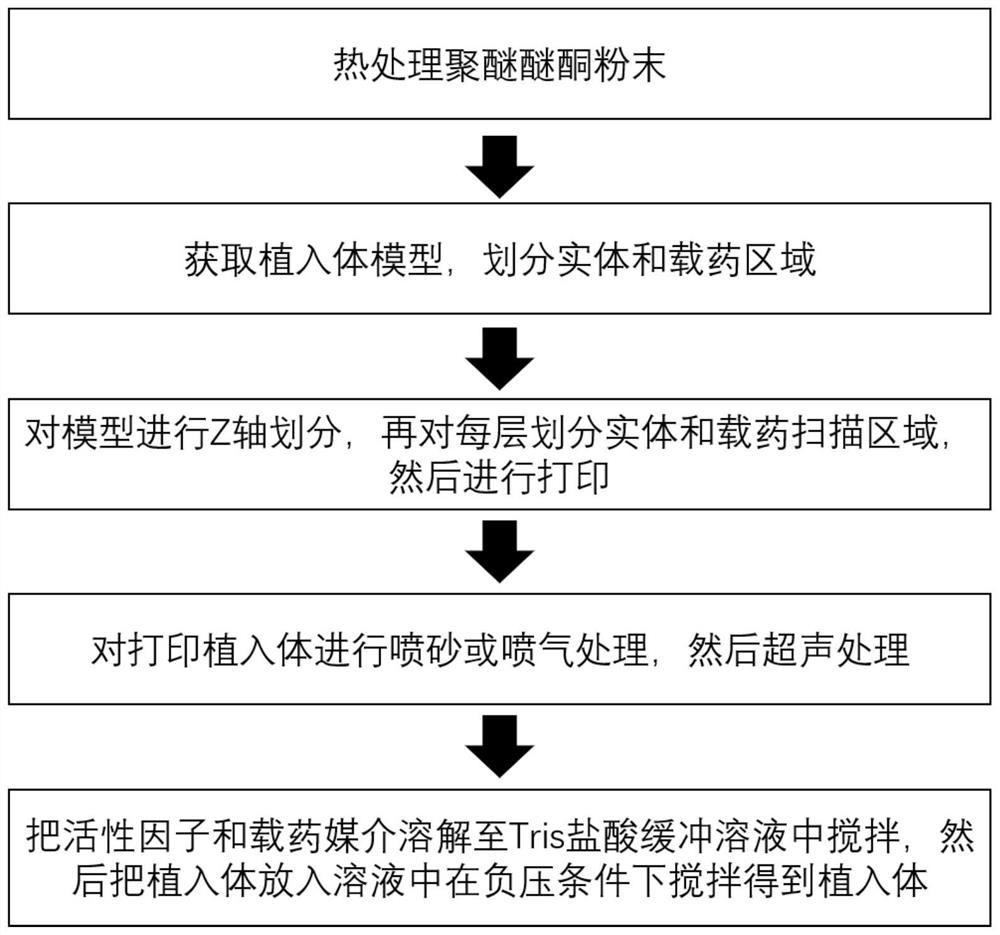
[0039] The present invention also provides a preparation method of a polyetheretherketone implant with controlled drug release, such as figure 1 As shown, the following steps are included:
[0040] (1) Select medical-grade polyether ether ketone, and conduct heat treatment to increase the sphericity of the powder.
[0041] (2) Acquire the model of the implant to be implanted, and divide the solid area and the drug-loaded area.
[0042] (3) Divide the model on the Z axis, set the layer thickness to 0.1mm, divide each layer into a physical scanning area and a drug-loaded scanning area according to the area divided in step (2), and set laser 1 to scan the physical scanning area, the laser 2 scans the drug-loaded scanning area, and sets the corresponding parameters. Then turn on the device for laser selective sintering printing. After printing, keep warm for 2 hours, then turn off the machine and cool down slowly.
[0043] (4) Perform sandblasting or air-jet treatment on the p...
Embodiment 1
[0068] This example provides a method for preparing a polyether ether ketone implant with controlled drug release, see figure 1 ,specifically:
[0069](1) Select 2 kg of medical-grade polyether ether ketone powder with a particle size of 30-70 μm, put it into an oven and heat-treat it in an argon atmosphere at 290° C. for 6 hours.
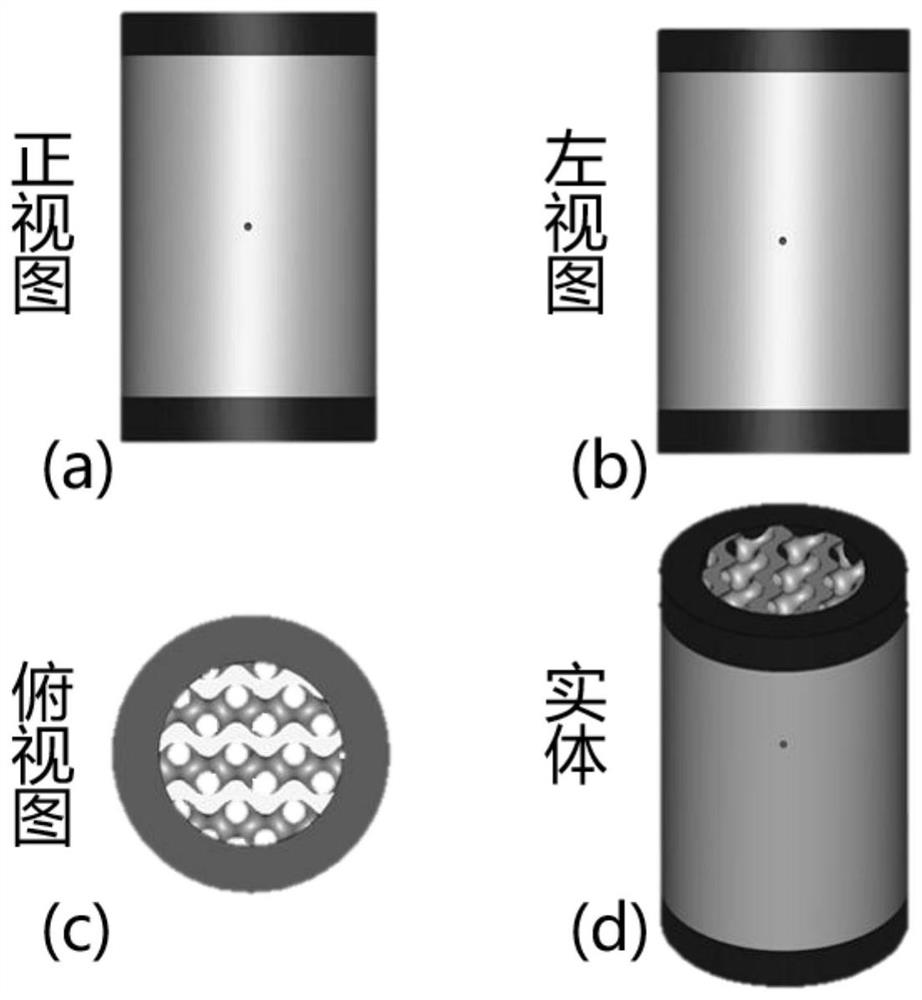
[0070] (2) The implanted body in this embodiment is an artificial vertebral body implanted in the cervical spine. According to the stress situation after numerical simulation, the entire vertebral body is divided into a drug-loaded area and a solid area, such as Figure 4 As shown in (a)-(c), it is exported as an STL format file on the "Magics" software.
[0071] (3) Process the imported STL file in the laser selective sintering equipment, and perform Z-axis division. Set each layer to 0.1mm, and divide each layer into a physical scanning area and a drug-loaded scanning area according to the area divided in step (2), set laser 1 to scan the physi...
Embodiment 2
[0078] This example provides a method for preparing a polyetheretherketone implant with controlled drug release, specifically:
[0079] (1) Select 2 kg of medical-grade polyether ether ketone powder with a particle size of 30-70 μm, put it into an oven and heat-treat it in an argon atmosphere at 250° C. for 10 h.
[0080] (2) The implant body of this embodiment is an artificial vertebral body implanted in the cervical spine, and the shape of the artificial vertebral body is designed, such as image 3 As shown in (a)-(d), according to the stress after numerical simulation, the whole vertebral body is divided into drug-loaded area and solid area, such as Figure 4 As shown in (a)-(c), it is exported as an STL format file on the "Magics" software.
[0081] (3) Process the imported STL file in the laser selective sintering equipment, and perform Z-axis division. Set each layer to 0.1mm, and divide each layer into a physical scanning area and a drug-loaded scanning area according...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Corner of repose | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com