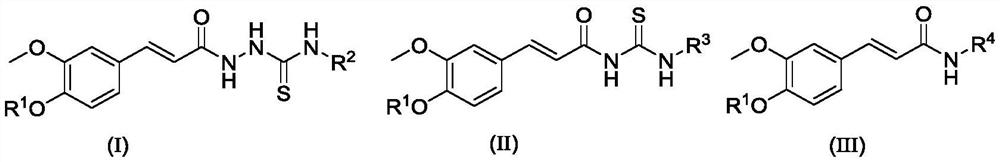
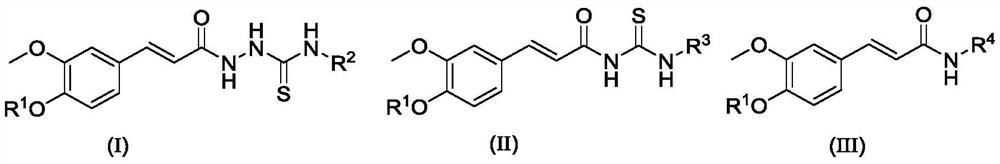
Amide-containing ferulic acid derivative as well as preparation method and application thereof
A technology of ferulic acid and derivatives, applied in the field of amide-containing ferulic acid derivatives and their preparation, can solve the problems of low antiviral activity, single structure, low anti-CMV activity of ferulic acid derivatives, etc. Antiviral activity, simple preparation process and stable physical and chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] (E)-2-(3-(allyloxy)-3-methoxyphenyl)acryloyl)-N-(4-(trifluoromethyl)phenyl)hydrazine-1-carbon thioamide (compound number is I1) preparation method, comprises the following steps:
[0081]
[0082] (1) Preparation of trans methyl ferulate
[0083] In a 250mL three-necked flask, add trans-ferulic acid (20.00g, 102.99mmoL) and 100mL of anhydrous methanol solution, after stirring at room temperature for 5min, slowly add concentrated H 2 SO 4 (10.10g, 102.99mmoL), the temperature was raised to 50°C. After the reaction was completed, the methanol solution was removed under reduced pressure, and then 40 mL of water was added to the system, which was adjusted with saturated sodium bicarbonate solution until no bubbles were generated. Finally, it was extracted three times with dichloromethane, and the organic phases were combined and concentrated under reduced pressure to obtain 19.08 g of a viscous liquid with a yield of 88.9%.
[0084] (2) Preparation of (E)-3-(4-(allyl...
Embodiment 2
[0093] (E)-2-(3-(4-ethoxy-3-methoxyphenyl)acryloyl)-N-(3-(methylthio)propyl)hydrazine-1-carbon thioamide ( Compound number is the preparation method of II 2), comprising the following steps:
[0094] Step (1)~(4) is the same as embodiment 1
[0095] (5) Add (E)-3-(4-ethoxy-3-methoxyphenyl)acrylohydrazide (0.50g, 2.12mmoL), 1-isothiocyanato - 4-toluene (0.31g, 2.12mmoL) and dry ethanol solution, stirred at room temperature. After the reaction, add saturated sodium bicarbonate solution to the system, use dichloromethane to extract three times 30mL each time, combine the organic phases, and use anhydrous sodium sulfate to dry, concentrate under reduced pressure and go through silica gel column chromatography, with petroleum ether / Ethyl acetate=3:1 (V / V) was used as the eluent for purification to obtain 0.19 g of white solid with a yield of 49.5%.
Embodiment 3
[0097] (E)-2-(3-(allyloxy)-3-methoxyphenyl)acryloyl)-N-(4-(trifluoromethyl)phenyl)hydrazine-1-carbon thioamide (the compound number is II1) preparation method, comprises the following steps:
[0098]
[0099] (1) Preparation of trans methyl ferulate
[0100] In a 250mL three-necked flask, add trans-ferulic acid (20.00g, 102.99mmoL) and 100mL of anhydrous methanol solution, after stirring at room temperature for 5min, slowly add concentrated H 2 SO 4 (10.10g, 102.99mmoL), the temperature was raised to 50°C. After the reaction was completed, the methanol solution was removed under reduced pressure, and then 40 mL of water was added to the system, which was adjusted with saturated sodium bicarbonate solution until no bubbles were generated. Finally, it was extracted three times with dichloromethane, and the organic phases were combined and concentrated under reduced pressure to obtain 19.08 g of a viscous liquid with a yield of 88.9%.
[0101] (2) Preparation of (E)-3-(4-i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com