Method for preparing cobalt-based nanosheet self-supporting electrode from selenium-containing ligand and application of cobalt-based nanosheet self-supporting electrode
A technology of self-supporting electrodes and nanosheets, applied in electrodes, nanotechnology, nanotechnology, etc., can solve problems such as unsatisfactory catalytic activity, achieve continuous water splitting stability, simple preparation method, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
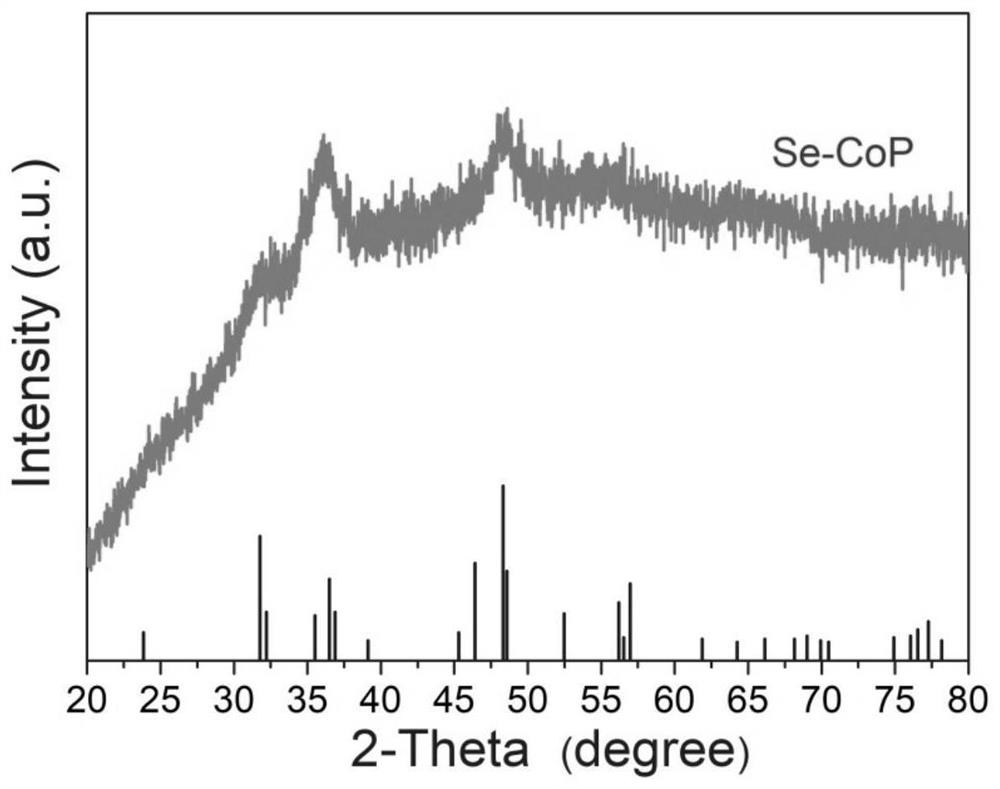
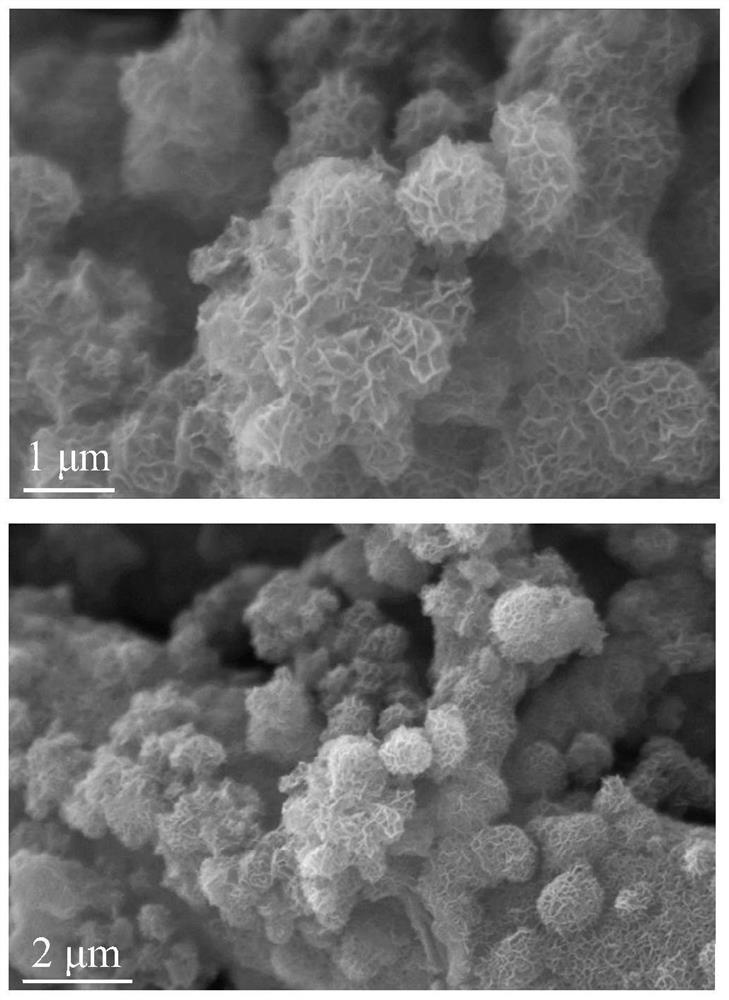
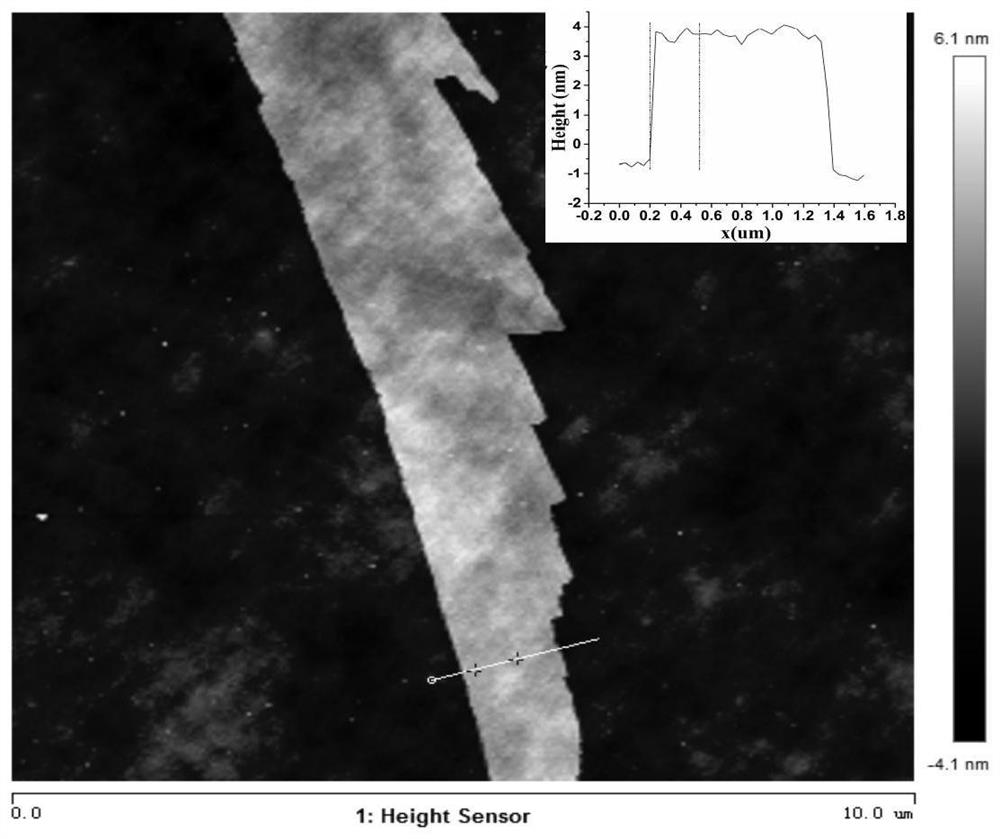
[0037] Example 1 Preparation of Se-CoP
[0038] To remove the oxide layer on the surface of the self-supporting electrode, the self-supporting electrode was sonicated in ethanol and acetone for 15 min, respectively, and then heated in HNO at 90 °C. 3 (6 M) solution for 1 h, then washed with ultrapure water until neutral, and transferred to a vacuum oven for drying. The selenium-containing ligand—bis(3,5-dimethyl-1H-pyrazol-4-yl)selenium H 2 Sebmpz and cobalt salts were dissolved in DMF solution to form a clear solution. The clean self-supporting electrode substrate is then quickly dipped into the mixed solution. After reacting at 160 °C for 6 to 24 h under stirring, the self-supporting electrode was taken out and washed twice with DMF solution, and dried in a vacuum oven at 60 °C. Place the porcelain boat containing the dried Se-Co-PCP self-supporting electrode in the center of the tube furnace and fill it with NaH 2 PO 2 The porcelain boat is placed upstream. Put the sa...
Embodiment 2
[0039] Example 2 Electrocatalytic HER performance test of Se-CoP self-supporting electrode
[0040] The electrocatalytic HER / performance test of the Se-CoP self-supporting electrode obtained in Example 1 was carried out on a CHI 760E electrochemical workstation (Shanghai Chenhua, China), using a traditional three-electrode system, and the electrolyte was 1.0 M KOH aqueous solution. Self-supporting electrodes, carbon rods and Hg / HgO were used as working, counter and reference electrodes, respectively. It can be seen from the figure that Se-CoP drives 5000 mA cm on the self-supporting electrode -2 and 1000 mA·cm -2 The overpotentials required for large current densities are 117 mV and 126 mV, respectively. Figure 7 The Tafel plot shown is from Figure 6 Calculated, it can be seen that the Tafel slope of Se-CoP on the self-supporting electrode is 62mV·dec -1 . In addition, at 500 mA·cm -2 The Se-CoP self-supporting electrode also exhibited sustained HER stability during el...
Embodiment 3
[0041] Example 3 Electrocatalytic OER performance test of Se-CoP self-supporting electrode
[0042] The electrocatalytic OER performance test of the Se-CoP self-supporting electrode obtained in Example 1 was carried out on a CHI 760E electrochemical workstation (Shanghai Chenhua, China), using a traditional three-electrode system, and the electrolyte was 1.0 M KOH aqueous solution. Self-supporting electrodes, Pt sheets and Hg / HgO were used as working, counter and reference electrodes, respectively. It can be seen from the figure that Se-CoP drives 5000 mA cm on the self-supporting electrode -2 and 1000 mA·cm -2 The overpotentials required for large current densities are 347 mV and 360 mV, respectively. Figure 10 The Tafel plot shown is from Figure 9 It is calculated that the Tafel slope of Se-CoP on the self-supporting electrode is 57mV·dec -1 . In addition, at 500 mA·cm -2 The Se-CoP self-supporting electrode also exhibited sustained OER stability during electrolysis ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com