Synthesis method of 3-methoxypropylamine
A technology for the synthesis of methoxypropylamine and its synthesis method, which is applied in the field of synthesis of 3-methoxypropylamine, can solve the problems of low yield of main product, increased production cost, increased processing difficulty, energy consumption and material consumption, etc., and achieves reduction of methanol consumption , improve the conversion rate and storage time, and improve the effect of selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] A kind of synthetic method of 3-methoxypropylamine, comprises the following steps:
[0027] (1) Using acrylonitrile and methanol as raw materials and an alkaline buffer as an auxiliary agent, acrylonitrile is added dropwise to a methanol solution containing an alkaline buffer to react to synthesize the crude product of 3-methoxypropionitrile;
[0028] (2) Using the crude product of 3-methoxypropionitrile as a raw material, in the presence of a solvent and a catalyst, the crude product of 3-methoxypropylamine is synthesized by catalytic hydrogenation.
[0029] (3) Carry out rectification to the 3-methoxypropylamine crude product, obtain the 3-methoxypropylamine product of purity>99.0%, and reclaim solvent methanol.
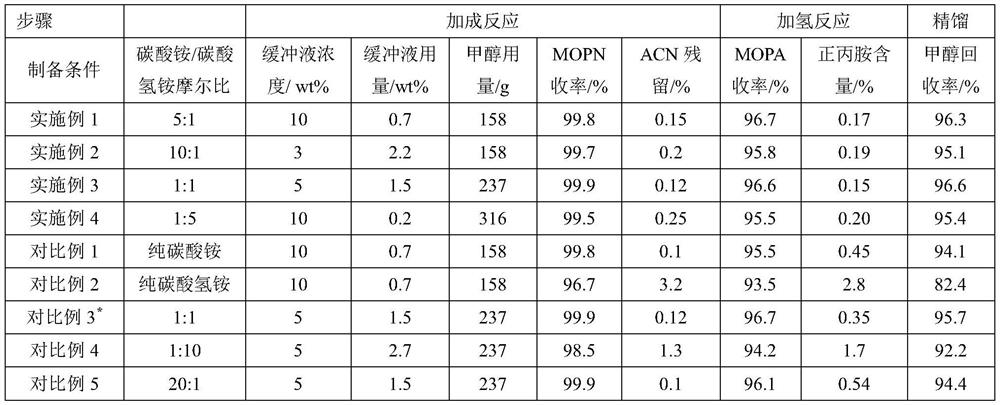
[0030] In the step (1), the alkaline buffer is ammonium carbonate-ammonium bicarbonate aqueous solution, and the total mass concentration of ammonium carbonate / ammonium bicarbonate is 0.1-10wt%, for example including but not limited to 0.1wt%, 0.5wt%, 1wt% ...
Embodiment 1
[0043] A kind of synthetic method of 3-methoxypropylamine, comprises the following steps:
[0044] (1) Weigh 0.096g of ammonium carbonate and 0.016g of ammonium bicarbonate and dissolve them in 1g of deionized water, combine them with 158g of methanol, and then transfer them to a 1L reaction kettle. After the kettle is combined, replace it with nitrogen three times, and then pressurize the nitrogen to 0.5MPa. Turn on stirring and heating. After the temperature in the reaction kettle rises to 35°C, use a convection pump for 0.15h -1 Acrylonitrile was fed at volumetric space velocity, and the reaction temperature was maintained at 35°C. When the feed amount of acrylonitrile reaches 174.5 g, the feed is stopped, the temperature is maintained and the reaction is prolonged for 1 h to finally obtain the crude product of 3-methoxypropionitrile. According to GC chromatographic analysis, the residual amount of acrylonitrile in the reaction solution was 0.15%, and the yield of 3-metho...
Embodiment 2
[0048] (1) Weigh 0.096g of ammonium carbonate and 0.008g of ammonium bicarbonate and dissolve them in 3.4g of deionized water, combine them with 158g of methanol and transfer them to a 1L reactor. After the reactor is combined, replace it with nitrogen three times, and then pressurize the nitrogen to 0.5MPa. Turn on stirring and heating. After the temperature in the reactor rises to 30°C, use a convection pump to -1 Acrylonitrile was fed at space velocity and the reaction temperature was maintained at 30°C. When the feed amount of acrylonitrile reaches 174.5 g, the feed is stopped, the reaction temperature is maintained and the reaction is prolonged for 1 h. Finally, the crude product of 3-methoxypropionitrile was obtained. According to GC chromatographic analysis, the residual amount of acrylonitrile in the reaction solution was 0.2%, and the yield of 3-methoxypropionitrile was 99.7%.
[0049] (2) The Raney cobalt catalyst with a wet weight of 2g is transferred to the auto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com