Preparation method of dehydroepiandrosterone acetate
A technology for dehydroepiandrosterone acetate and dienolone acetate, which is applied in the field of preparation of dehydroepiandrosterone acetate, and can solve the problems of restricting production efficiency, difficulty in realizing one-pot method, large amount of solvent and waste water, etc. , to achieve the effects of shortening the preparation cycle, reducing the difficulty of process control and increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
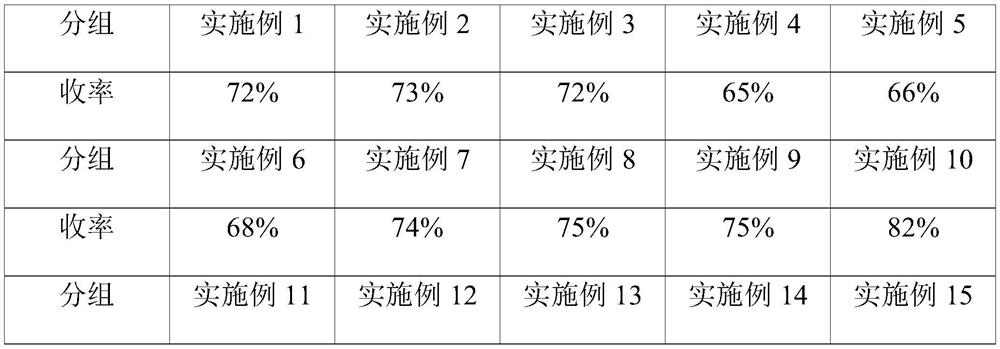
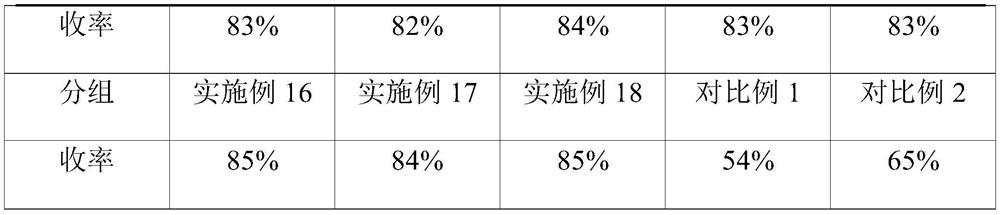
Embodiment 1
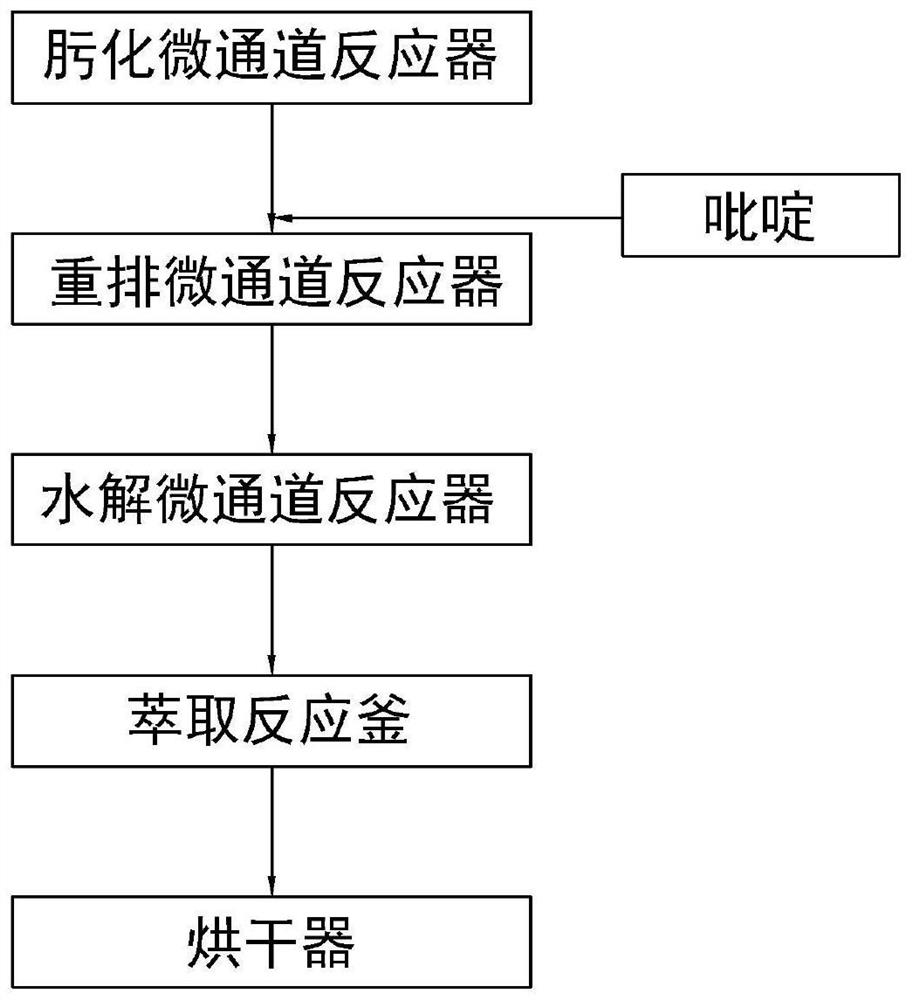
[0033] Configure hydroxylamine hydrochloride solution: mix hydroxylamine hydrochloride and pyridine according to the weight ratio of 1:10, stir evenly to obtain hydroxylamine hydrochloride solution;
[0034]Configure the acetic acid gestational dienolone solution: mix the acetic acid gestational dienolone and pyridine according to the weight ratio of 1:0.5, and stir evenly to obtain the acetic acid gestational dienolone solution;
[0035] Pump the hydroxylamine hydrochloride solution and the dienolone acetic acid solution into the oximation microchannel reactor at a volume ratio of 1:0.2, keep the temperature in the oximation microchannel reactor at 45-55°C, feed the reaction solution The residence time is 60s, and the oximation reaction solution is obtained;
[0036] Cool the oximation reaction solution to 30°C, pump it into the rearrangement microchannel reactor, the inner wall of the oximation microchannel reactor is loaded with titanium tetrachloride, and keep the temperat...
Embodiment 2
[0040] On the basis of Example 1, other parameters remain unchanged, the mass ratio of hydroxylamine hydrochloride and pyridine in the hydroxylamine hydrochloride solution is 1:15, the mass ratio of acetic acid gestational dienolone and pyridine in the acetic acid gestational dienolone solution 1:1, the volume ratio of hydroxylamine hydrochloride solution and acetic acid pregnant dienolone solution is 1:0.4, the volume flow rate ratio of rearrangement reaction solution and hydrochloric acid solution is 1:0.2, the mass concentration of hydrochloric acid is 25%, the extraction solvent for toluene.
Embodiment 3
[0042] On the basis of Example 1, other parameters remain unchanged, the mass ratio of hydroxylamine hydrochloride and pyridine in the hydroxylamine hydrochloride solution is 1:20, the mass ratio of acetic acid gestational dienolone and pyridine in the acetic acid gestational dienolone solution 1:2, the volume ratio of hydroxylamine hydrochloride solution and acetic acid pregnant dienolone solution is 1:0.6, the volume flow rate ratio of rearrangement reaction solution and hydrochloric acid solution is 1:0.3, the mass concentration of hydrochloric acid is 30%, the extraction solvent to dichloromethane.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com