MRNA (messenger ribonucleic acid) tumor vaccine for improving DCs (dendritic cells) disability in tumor immune microenvironment as well as preparation method and application of mRNA tumor vaccine
A tumor vaccine and microenvironment technology, applied in the field of mRNA tumor vaccine preparation for improving the incapacitation of tumor immune microenvironment DCs, can solve the problems of complex production process, weak immunogenicity, etc., and achieve improved inhibitory effect, strong application value, The effect of activated CTL activation and proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
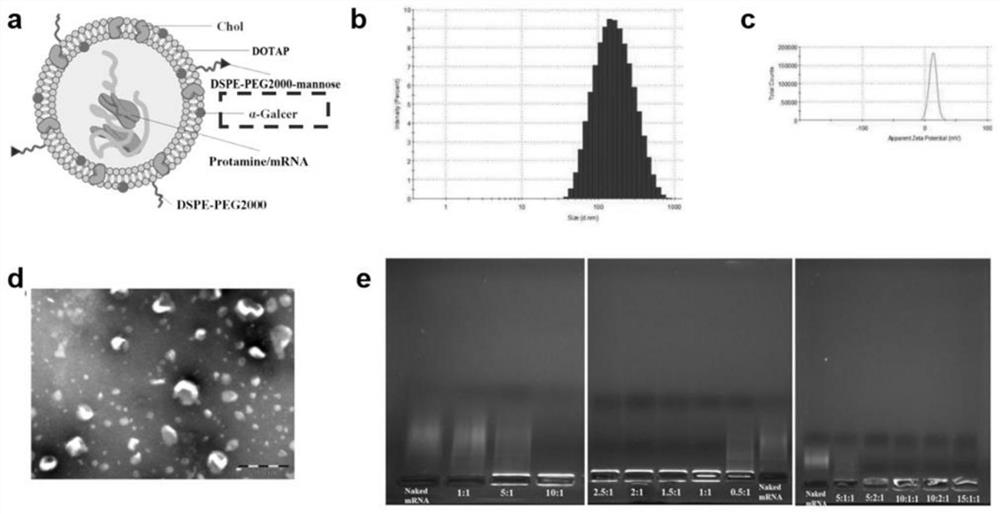
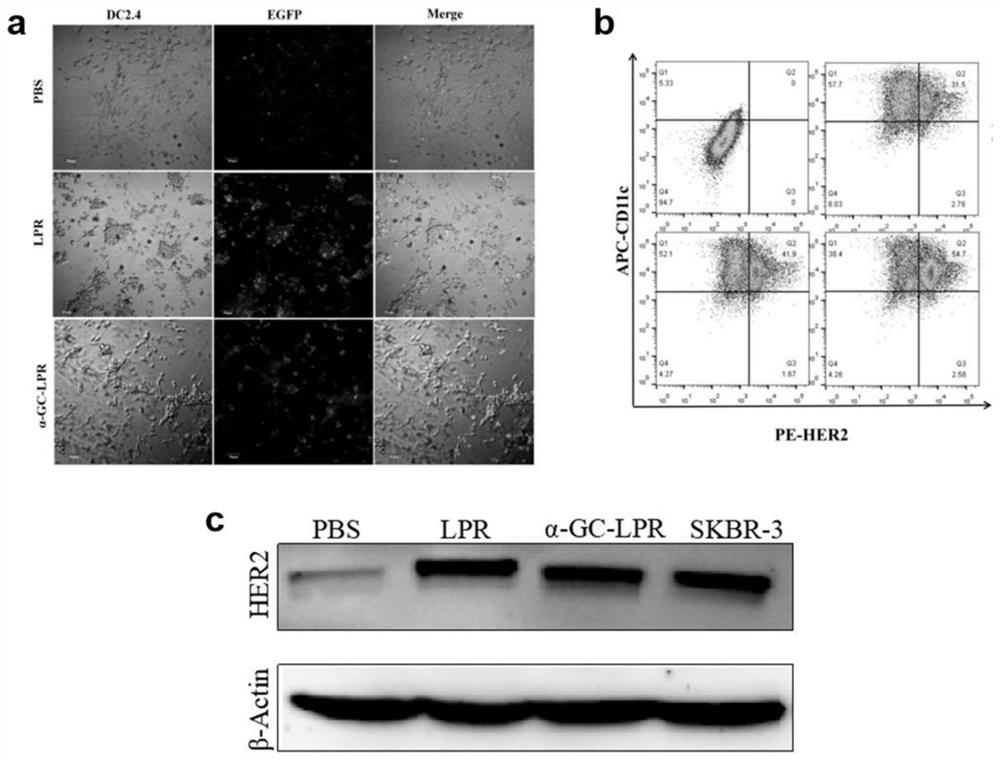
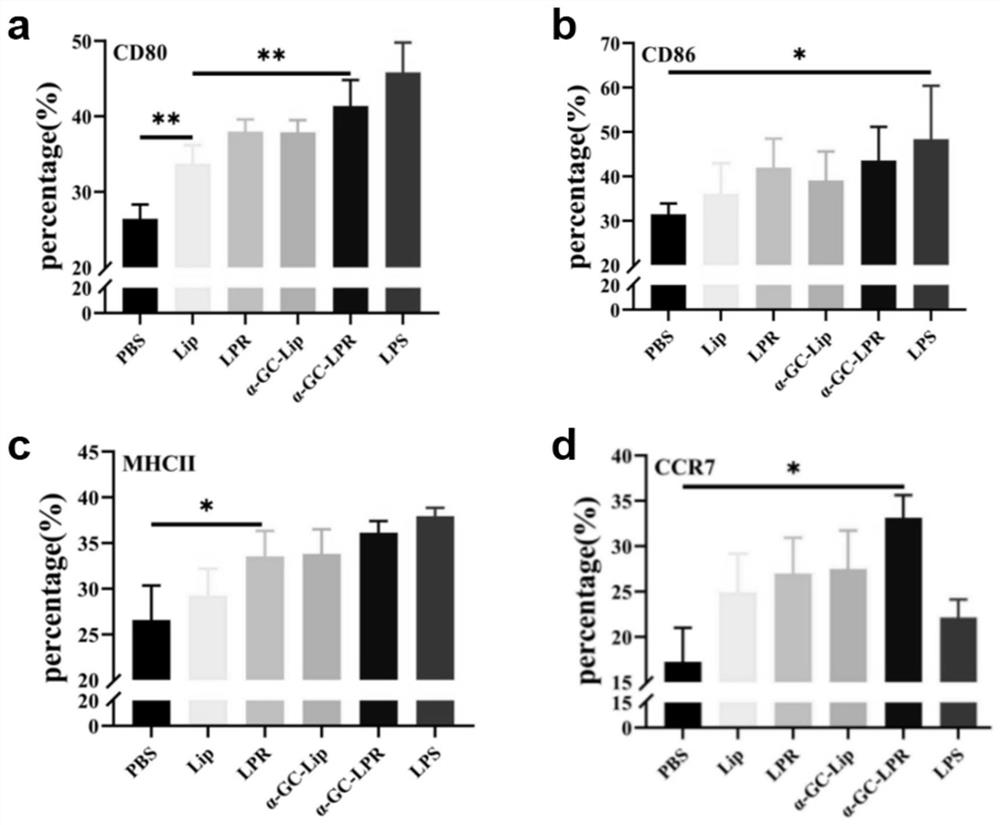
[0049] Example 1: A thin-film dispersion method was used to prepare a cationic complex mRNA tumor vaccine that can improve the inability of DCs in the tumor immune microenvironment.
[0050] Dissolve DDAB, Chol, DSPE-PEG2000, DSPE-PEG-mannose, and α-Galcer in 3ml chloroform at a molar ratio of 2:2:0.1:0.05:0.3, add to a 100ml round bottom flask, and place on a vortex mixer After vortex mixing for 1 min, 37°C water bath rotary evaporation for 15 min, and drying under reduced pressure to obtain a cationic liposome film. Nitrogen gas was passed into the round bottom flask where the cationic liposome film was formed, and the organic solvent was evaporated to dryness. Add pH 7.4 Tris buffer and hydrate in a water bath at 50°C for 20 minutes to obtain a blank cationic liposome solution (Lip) with a nm size of 80-600 nm. Incubate at room temperature for 10 min at a protamine:mRNA mass ratio of 1:1 to obtain a protamine-HER2 mRNA condensate. Then it was added to Lip and incubated fo...
Embodiment 2
[0052] Example 2: A thin-film dispersion method was used to prepare a cationic complex mRNA tumor vaccine that can improve the dysfunction of DCs in the tumor immune microenvironment.
[0053] Dissolve DC-Chol, Chol, DSPE-PEG2000, DSPE-PEG-mannose, and α-Galcer in 3ml of chloroform at a molar ratio of 2:3:0.3:0.1:0.5, add to a 100ml round bottom flask, and vortex to mix After vortex mixing on a device for 1 min, 37°C water bath rotary evaporation for 15 min, and drying under reduced pressure to obtain a cationic liposome film. Nitrogen gas was passed into the round bottom flask where the cationic liposome film was formed, and the organic solvent was evaporated to dryness. Add pH 7.4 HEPES buffer solution, and hydrate in a water bath at 45°C for 20 minutes to obtain a blank cationic liposome solution (Lip) of 80-600 nm. Incubate at room temperature for 10 min at a protamine:mRNA mass ratio of 1:1 to obtain a protamine-HER2 mRNA condensate. Then it was added to Lip and incubat...
Embodiment 3
[0055] Example 3: A thin-film dispersion method was used to prepare a cationic complex mRNA tumor vaccine that can improve the inability of DCs in the tumor immune microenvironment.
[0056]Dissolve DOTAP, Chol, DSPE-PEG2000, DSPE-PEG-mannose, and α-Galcer in 3ml chloroform at a molar ratio of 2:2:0.3:0.05:0.5, add to a 100ml round bottom flask, and place on a vortex mixer After vortex mixing for 1 min, 37°C water bath rotary evaporation for 20 min, and drying under reduced pressure to obtain a cationic liposome film. Nitrogen gas was passed into the round bottom flask where the cationic liposome film was formed, and the organic solvent was evaporated to dryness. Add pH 7.4 Tris buffer and hydrate in a water bath at 50°C for 20 minutes to obtain a blank cationic liposome solution (Lip) with a nm size of 80-600 nm. Incubate at room temperature for 10 min at a protamine:mRNA mass ratio of 1:1 to obtain a protamine-HER2 mRNA condensate. Then it was added to Lip and incubated fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Electric potential | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com