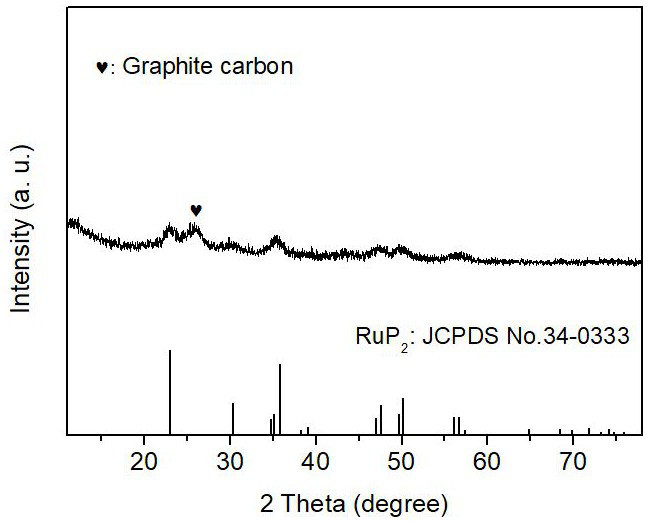
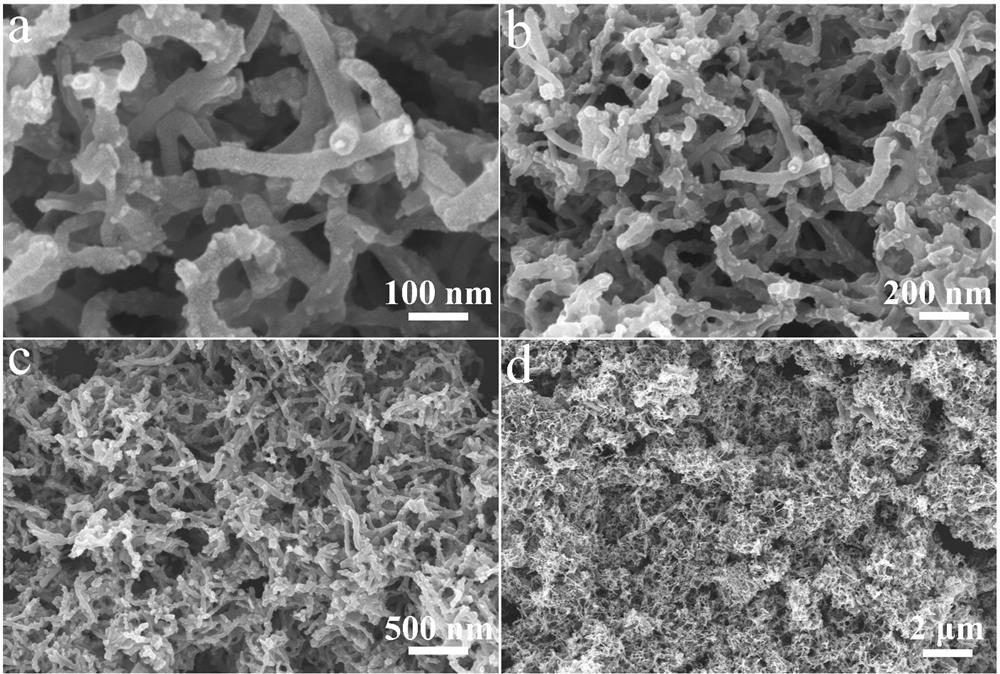
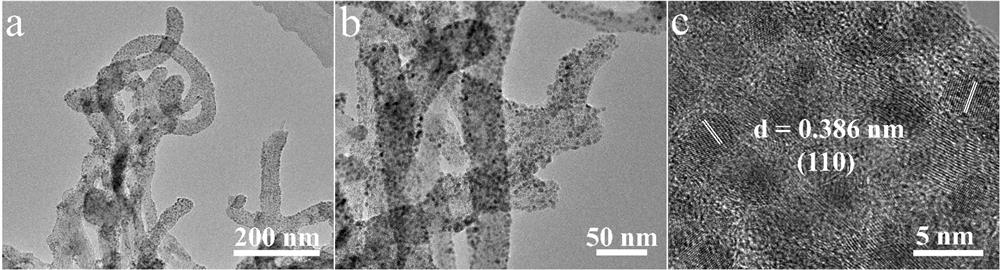
Synthesis method of superfine ruthenium diphosphide nanoparticle electrocatalyst
A technology of fine ruthenium diphosphide and nanoparticles, applied in electrodes, electrolysis process, electrolysis components, etc., can solve the problem of reduction of catalytic active sites, and achieve the effect of reducing a large amount of release and excellent catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 RuP 2 Preparation of @NPS-CNT catalyst
[0032] 100 mg of carbon nanotubes (outer diameter: 15 nm, length: 50 μm) and ruthenium trichloride RuCl 3 (0.2 mmol) was added into 80 mL ultra-dry methanol, ultrasonicated for 1 h, and made into A solution. Dissolve 300 mg of hexachlorotripolyphosphazene and 675 mg of 4,4'-dihydroxydiphenylsulfone in 20 mL of ultra-dry methanol to form solution B, add the obtained solution of B to the above 80 mL of LA solution, and stir at room temperature for 10 After min, make C solution. Then, 1 mL of triethylamine was added dropwise to the above solution C, and after stirring at room temperature for 24 h, it was filtered with organic polymer membrane, washed three times with ordinary methanol, and then dried naturally to obtain the Ru-PZS@CNT precursor. Subsequently, the obtained Ru-PZS@CNT precursor powder was placed in a porcelain boat and placed in a tube furnace. The tube furnace was heated to 800°C under Ar flow at a ramp...
Embodiment 2
[0033] Example 2 RuP obtained in Example 1 2 Electrocatalytic HER activity test of @NPS-CNT
[0034] The RuP of embodiment 1 gained 2 The electrocatalytic HER performance test of @NPS-CNT was carried out on a CHI760E electrochemical workstation. The electrolyte is 1.0 M KOH aqueous solution. Glassy carbon electrode, Ag / AgCl and graphite rod were used as working electrode, reference electrode and counter electrode, respectively. Figure 6 The linear sweep voltammetry graph shown was obtained at a sweep rate of 5.0 mV / s, obtained by Figure 6 It can be seen that RuP 2 @NPS-CNT drives 10, 100 and 300 mA cm -2 The required overpotentials for the current densities are 10, 83, and 158 mV, respectively, and the catalytic activity is significantly better than that of commercial Pt / C. Figure 7 The Tafel plot shown is from Figure 6 Calculated, it can be known that RuP 2 @NPS-CNT has a Tafel slope of 22 mV dec -1, much lower than commercial Pt / C.
Embodiment 3
[0035] Example 3 RuP obtained in Example 1 2 Electrochemical stability test of @NPS-CNT
[0036] Figure 8 The potentiostatic electrolysis diagram shown is for controlling the RuP 2 @NPS-CNT was obtained by electrolysis for 25 h at overpotentials of 10, 83 and 158 mV, from which it can be seen that the RuP 2 @NPS-CNT maintains good catalytic stability at different current densities. Figure 9 RuP shown 2 @NPS-CNT The linear sweep voltammetry curve before and after 10,000 CV scans, the two lines basically overlap, indicating RuP 2 The @NPS-CNT catalyst has good catalytic stability.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com