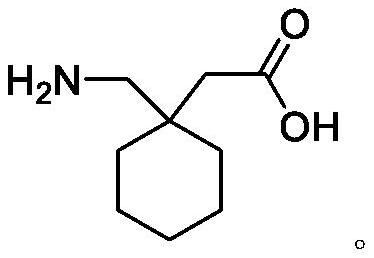
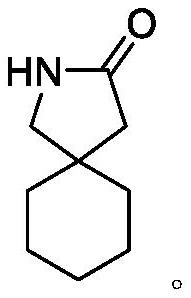
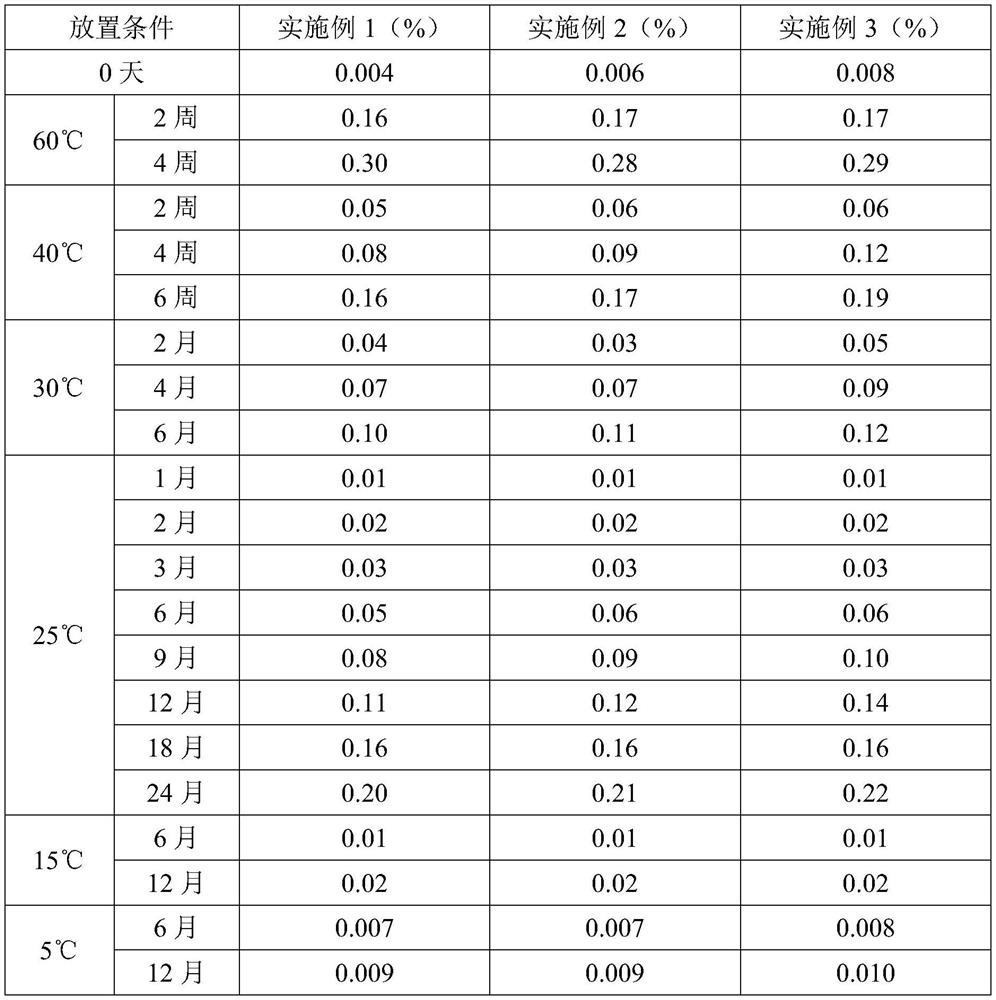
TPGS micelle oral liquid containing gabapentin compound and preparation method of TPGS micelle oral liquid
The technology of micellar oral liquid and gabapentin is applied in the direction of medical preparations containing active ingredients, medical preparations with inactive ingredients, and pharmaceutical formulas, which can solve the problems of short validity period and achieve the reduction of lactam formation and masking Bitter taste, effect of improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Gaboba Spirit Laurel Subto Micell Oral Liquid
[0029] Step 1 Complex Preparation: 20.0 g (0.1 mol) of lauric acid and 5 g (0.03 mol) gabetry were fully dissolved in 50 ml dimethyl sulfoxide, and the room temperature was allowed to stand for 3 days, i.e., Gabba-biling lauric acid complex solution.
[0030]Step 2 Composite micelle prepares: 50.0 g of polyethylene glycol 1000 vitamin E succinate in the gabbar sputum lauric acid composite solution, after which dimethyl sulfoxide is removed from the dialysis apparatus to obtain a drug micelle solution.
[0031] Step 3 Composite micelle olet oral is prepared: referred to the xylitol 25g, orange fragrance 0.05 g, potassium sorbate 0.1 g, dissolved in purified water 150 ml, and slowly mixed with step 2 under stirring The drug micelle solution is mixed evenly, and the polyethylene glycol 1000 vitamin E succinate gum is obtained by filtration to 200 mL, filtration, and filling.
Embodiment 2
[0032] Example 2: Gabobajikutic Composite Micell Oral Liquid
[0033] Step 1 Complex Preparation: 4.5 g (0.03 mol) of octanoic acid and 5 g (0.03 mol) gabetry is fully dissolved in 100 ml of acetone mixing liquid, and about 40 ° C for 1 day, i.e., gabbar sputum complex solution.
[0034] Step 2 Complex micelle prepares: 50.0 g of polyethylene glycol 1000 vitamin E succinate in the gabbar butty acid composite solution of the above step 1, and the solution is reduced by a solution in a solution in a cylinder bottle to obtain a uniform film. The addition of 100 ml of purified water was added, and the hydration was rotated in a water bath in the 37 ° C to obtain a drug micelle solution.
[0035] Step 3 Complex micelle olet oral hydrolysis: 20g of sorbitol, 0.01 g of banana flavor, 0.1 g of Nepo golden methyl ester, dissolved in 50 ml of purified water, stirred, slowly add the drug obtained in step 2 In the micelle solution, mix evenly, set to 200 mL, filtered, filling, and filling the...
Embodiment 3
[0036] Example 3: Gaboba sputum oleic acid composite micelle oral liquid
[0037] The complex of step 1 was prepared: 45.7 g (0.15 mol) of sodium oleate and 5 g (0.03 mol) gabetry were added to 100 ml of water well, and the solution solution pH to 3 to 6 of hydrochloric acid was transferred with a magnetic stirring of about 20 ° C. The core of the coabetry oleic acid complex solid.
[0038] Step 2 Composite micelle preparation: Step 1 from the elutable oleic acid complex and 50.0 g of polyethylene glycol 1000 vitamin E succinate, add 100 ml of water to 37 ° C ultrasonic hydration, and mixture is obtained Solution.
[0039] Step 3 Composite micelle oral is prepared: 10g of erythritol 10g, strawberry flavor 0.01 g, sodium benzoate 0.1 g to dissolve in 50 ml of purified water, stirred, slowly add the mixture of step 2 to the mixture of step 2 , Mix evenly, set to 200 mL, filtrate, and fill the micelle oral fluid of the Gabobarn-bipped complex.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com