Arendronic acid modified astragaloside mPEG-PLGA nano-micelle and research on anti-osteoporosis effect of alendronic acid modified astragaloside mPEG-PLGA nano-micelle
An anti-osteoporosis, astragaloside IV technology, applied in the field of pharmaceutical preparations, can solve the problems that the improvement of osteoporosis treatment has not yet been realized, achieve good bone tissue targeting ability, delay internal circulation time, and improve oral bioavailability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
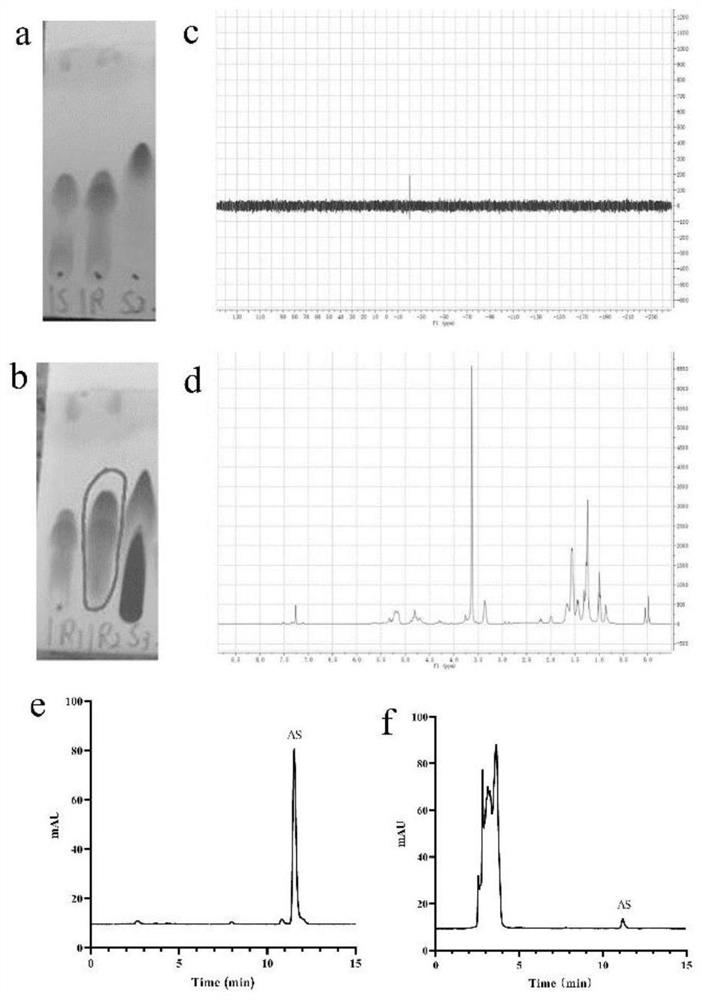
[0029] Synthesis of alendronate-modified mPEG-PLGA (AL-mPEG-PLGA) bone-targeting material in the study of alendronic acid-modified astragaloside IV mPEG-PLGA nanomicelles and its anti-osteoporosis effect:
[0030] MPEG2000-PLGA18000 (50 / 50) compound was dissolved in 2 mL of DMF solution, and CDI (2.5 mg, 0.015 mmol) was added while stirring. Reaction at room temperature for 5h. The reaction was monitored on a TLC plate with a mixture of dichloromethane and methanol. After the two compounds were reacted, the alendronate sodium trihydrate and tetrabutylammonium hydroxide solution (1:2 molar ratio) were stirred, added to the reaction solution, and reacted at 80° C. for 5 h. The reaction was monitored on a TLC plate using a mixture of dichloromethane and methanol. After the reaction, use a rotary evaporator to remove DMF at 70-80°C. The remaining reaction solution was washed with a small amount of water, and excess aluminum sodium and tetrabutylammonium hydroxide (supernatant) ...
Embodiment 2
[0033] Preparation of a kind of alendronic acid-modified astragaloside IV mPEG-PLGA nanomicelle:
[0034] The invention adopts a dialysis method to prepare astragaloside IV mPEG-PLGA nano micelles. Accurately weigh 10mg AL-mPEG-PLGA and dissolve in 10mL distilled water. After ultrasonic probe 30 times, 1.0 mg / mL AL-mPEG-PLGA blank micellar solution was obtained. Then, under magnetic stirring, gradually add 15% ethanol solution of astragaloside IV. After stirring for 10 min, the solution was transferred to a dialysis bag (MWCO 700,000000Da) and dialyzed against double distilled water for 24 h to remove ethanol. The dialysis product was centrifuged at 4000rpm / min for 10min to remove unencapsulated astragaloside IV. After the supernatant was freeze-dried, astragaloside IV mPEG-PLGA nanomicelle solid powder was prepared for follow-up research.
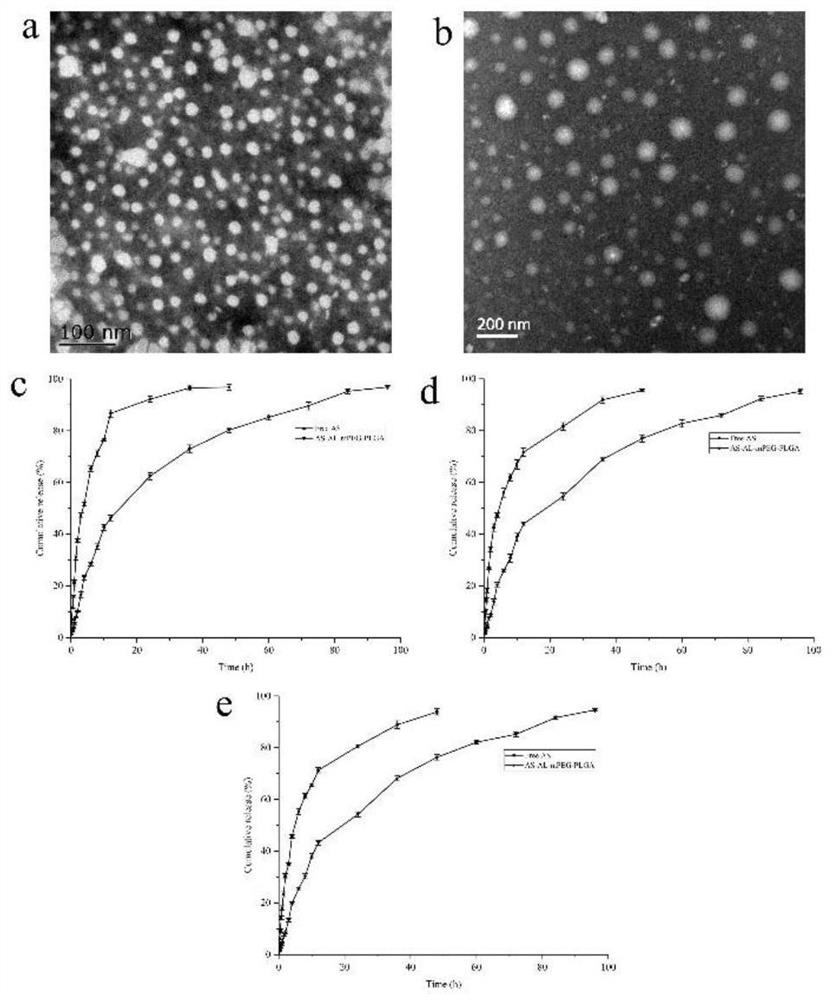
[0035] Physical characterization: After diluting the astragaloside IV mPEG-PLGA nanomicelles prepared in Example 2 with water, a part...
Embodiment 3
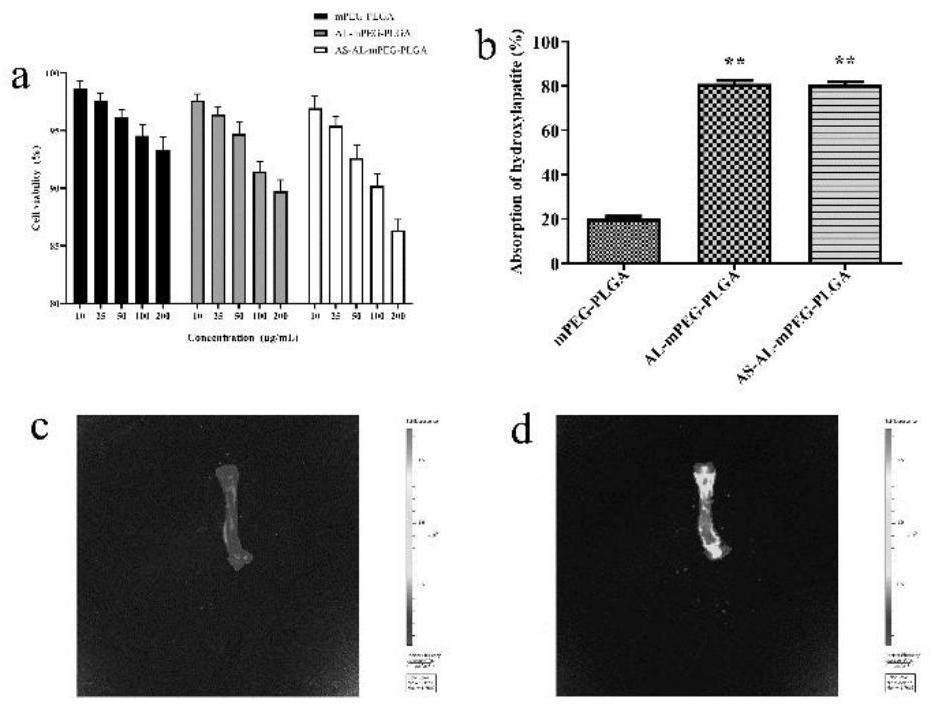
[0039] Cytotoxicity Study of Astragaloside IV mPEG-PLGA Nanomicelles in Vitro
[0040] The cytotoxicity of astragaloside IV mPEG-PLGA nanomicelles on MC3T3-E1 cells was detected by MTT assay. After the logarithmic growth phase, MC3T3-E1 cells were seeded in 96-well plates at a density of 5×104 per well, and then incubated at 37°C and 5% carbon dioxide for 24h. After the cells were completely iron-armed, different concentrations (10, 25, 50, 100 and 200 μg / mL) of AL-mPEG-PLGA blank micelles solution, astragaloside IV mPEG-PLGA nanomicelle solution and mPEG-PLGA blank micelles were added solution. At the same time, untreated blank cells were used as the control group, and after continuous culture for 48 hours, 20 μL of MTT solution (5 mg / mL) was added to each well. After continuing to culture in the incubator for 4 hours, discard the supernatant, add DMSO (100 μL) to each well, and shake at constant temperature for 20 minutes. Measure the absorbance at 570nm with a microplate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap