Novel preparation method of eslicarbazepine acetate
A technology of eslicarbazepine acetate and potassium carbonate, applied in the field of drug synthesis, can solve the problems of unstable yield of eslicarbazepine acetate, inability to obtain a product, complicated operation, etc., and achieves simplified technological process and operation time, The effect of low cost and optimized reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
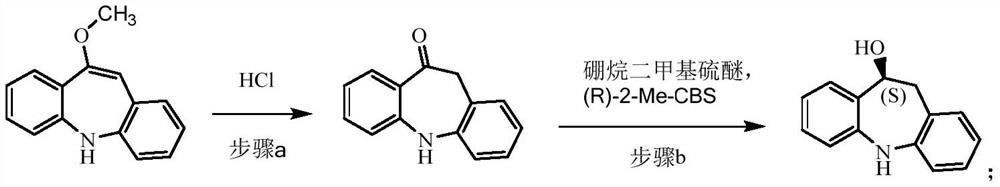
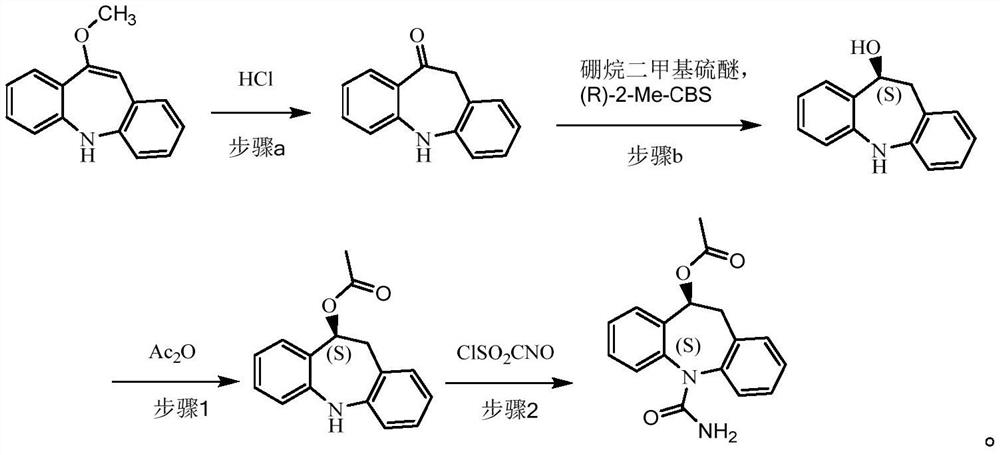
[0061] (1) Preparation of 10-carbonyliminostilbene
[0062] Add 880ml of methanol, 220ml of purified water, 220g of 10-methoxyiminostilbene, and 11ml of concentrated hydrochloric acid into the reaction flask, and heat up to 65°C-70°C for reaction. After the reaction was completed, the temperature was lowered to 25°C-30°C, 220ml of purified water was added, and stirring was continued for 1h after the addition. After the reaction is completed, filter, wash the filter cake with a large amount of water until it becomes neutral, and air-dry at 50° C. to obtain 200 g of a yellow powder.
[0063] (2) Preparation of (10S)-10-hydroxyiminostilbene
[0064] Under nitrogen protection, add 600ml of dichloromethane and 1.25mol of borane dimethyl sulfide into the reaction flask, cool down to -10°C to -5°C, add 38ml of (R)-2-methyl-CBS-oxazoboridine Toluene solution (1mol / L), add dropwise a mixed solution of 200g 10-carbonyliminostilbene and dichloromethane (1200ml), and control the tempera...
Embodiment 2
[0070] (1) Preparation of 10-carbonyliminostilbene
[0071] Add 660ml of methanol, 220ml of purified water, 220g of 10-methoxyiminostilbene, and 10ml of concentrated hydrochloric acid into the reaction flask, and heat up to 60°C-65°C for reaction. After the reaction was completed, the temperature was lowered to 20°C-25°C, 220ml of purified water was added, and stirring was continued for 1h after the addition. After the reaction was completed, filter, wash the filter cake with a large amount of water until it became neutral, and dry it with air at 50°C to obtain 198 g of yellow powder.
[0072] (2) Preparation of (10S)-10-hydroxyiminostilbene
[0073] Under nitrogen protection, add 600ml of dichloromethane and 1.25mol of borane dimethyl sulfide into the reaction flask, cool down to -10°C to -5°C, add 38ml of (R)-2-methyl-CBS-oxazoboridine Toluene solution (1mol / L), add dropwise the mixed solution of 198g 10-carbonyliminostilbene and dichloromethane (1200ml), dropwise process ...
Embodiment 3
[0079] (1) and (2) are the same as Comparative Example 1.
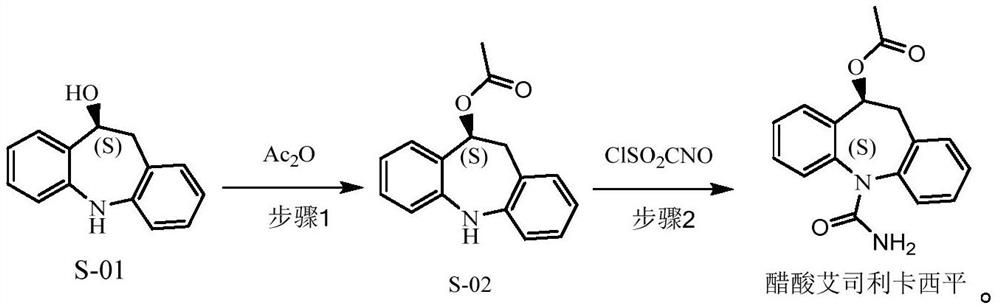
[0080] (3) Preparation of (10S)-10-acetyliminostilbene
[0081] Add 150g of (10S)-10-hydroxyiminostilbene, 750ml of dichloromethane, 170.0g of potassium carbonate, and 120.1g of acetic anhydride obtained in step (2) into the reaction flask, raise the temperature to about 50°C, that is, the solvent reflux state, and keep The reaction system was refluxed for 5h. After the reaction was completed, the temperature was lowered to 20° C., filtered, and the organic phase was washed successively with sodium bicarbonate (1 L), purified water (1 L), dried over magnesium sulfate, and filtered. The organic phase was directly used in the next reaction.
[0082] (4) synthesis of eslicarbazepine acetate
[0083] Add 840ml of dichloromethane into the reaction flask, cool down to -5°C to 5°C, add 130.0ml of chlorosulfonic acid isocyanate, control the temperature at -5°C to 5°C and stir for 10 minutes; drop the organic phase of the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com